Multi-dose oral abuse deterrent formulation of Loperamide using Hot melt extrusion
Loperamide, an over the counter anti-diarrheal drug, also infamously referred to as “poor man’s methadone”.
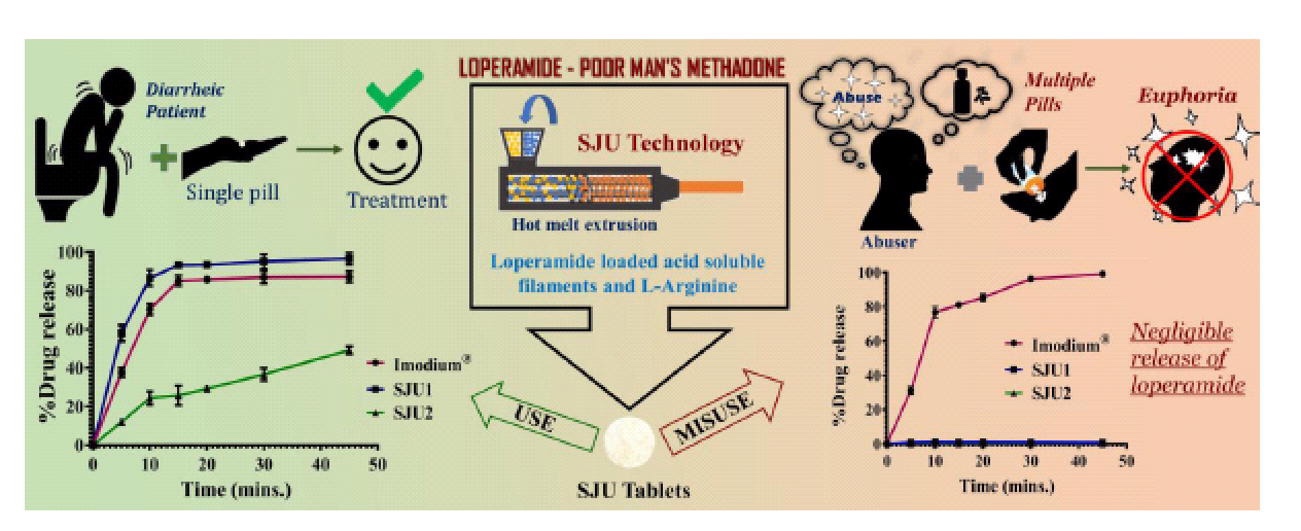
Due to the ease of availability and low price, people/patients abuse it by consuming more than 30 tablets to achieve euphoric effect and to combat opioid withdrawal. But supratherapeutic doses of loperamide result in severe respiratory depression, cardiac dysrhythmia and mortality. To address this issue, we developed a unique and innovative technology to deter multi-dose oral abuse.
The concept is to design a tablet which can immediate release loperamide in diarrheic patients (single tablet) while stops loperamide release in case of intentional multi-dose ingestion. Loperamide was molecularly dispersed into gastric soluble cationic polymers – Eudragit® EPO and Kollicoat® Smartseal 100P using hot melt extrusion to obtain filament. Filaments were milled and compressed into tablets ((Eudragit®EPO (SJU1) and Kollicoat® Smartseal (SJU2)) with optimized amount of L-Arginine.
Dissolution in 250 mL of Fasted state simulated gastric fluid (FaSSGF) revealed that single tablet of Imodium® (marketed formulation) and SJU1 showed >85% of release within 15 minutes. Most importantly, in multi-unit dissolution (15 tablets), Imodium® exhibited >90% release but SJU tablets showed <2% of drug release thus demonstrating its ability to deter multi-dose oral abuse. Read more

