Understanding the release performance of pellets with hydrophobic inclusions in sustained-release coating
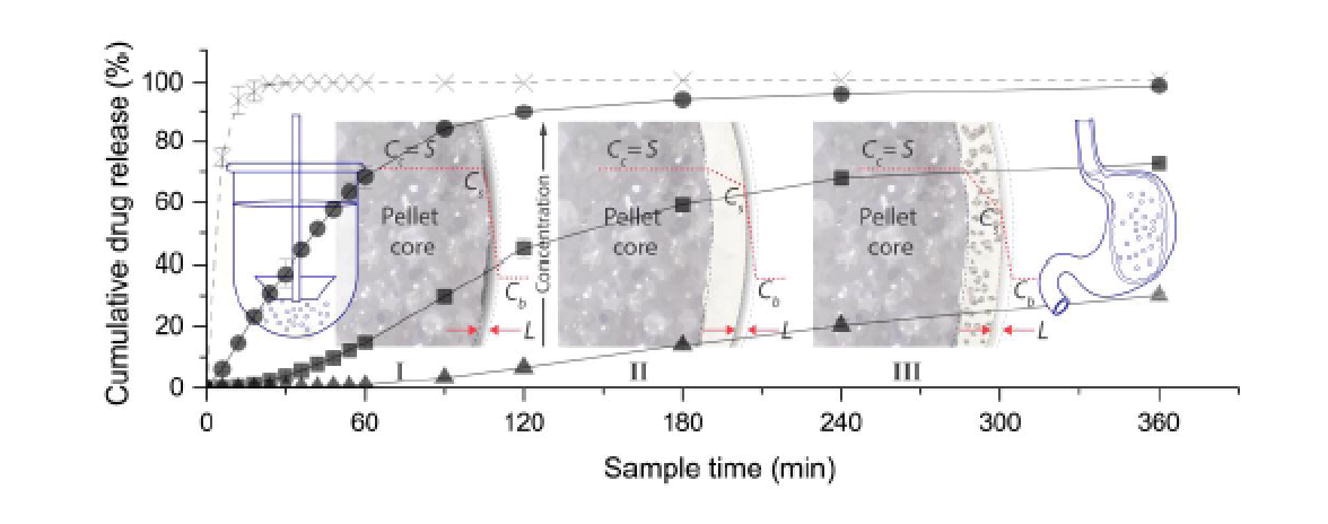
The aim of this study was to evaluate how the addition of hydrophobic inclusions to sustained-release pellet film coat can affect drug release. Sustained-release formulations, in particular multiparticulate systems are gaining popularity because they are able to reduce the dosing frequency of drugs that require multiple dosing. In addition, the risk of dose dumping is low and local gastrointestinal irritation is minimised. Metformin-loaded pellets, prepared via extrusion-spheronisation, were coated with ethyl cellulose (EC)-based film coat, with and without hydrophobic inclusions to a series of coat thickness. Stearic acid 50 (SA) and hydrogenated castor oil (HCO) were the hydrophobic inclusions used. Drug release was investigated using the USP dissolution apparatus 2 and an ultraviolet imager. Release kinetics were analysed using the zero-order model. The physical properties of the pellets were characterised before and after dissolution. The addition of hydrophobic inclusions to EC-based film coat slowed down drug release, with SA slowing down drug release more than HCO. The influence of hydrophobic inclusions on drug release was clearly observable when the pellets were coated to 10 % weight gain. It was postulated that the hydrophobic inclusions acted as physical barriers to increase the tortuosity of the diffusional path through the pellet film coat. The use of hydrophobic inclusions to control the rate of drug dissolution was shown to be promising. This could translate into potential cost and time savings with less materials and time used.

