The applicability of pharmaceutical polymeric blends for the fused deposition modelling (FDM) 3D technique
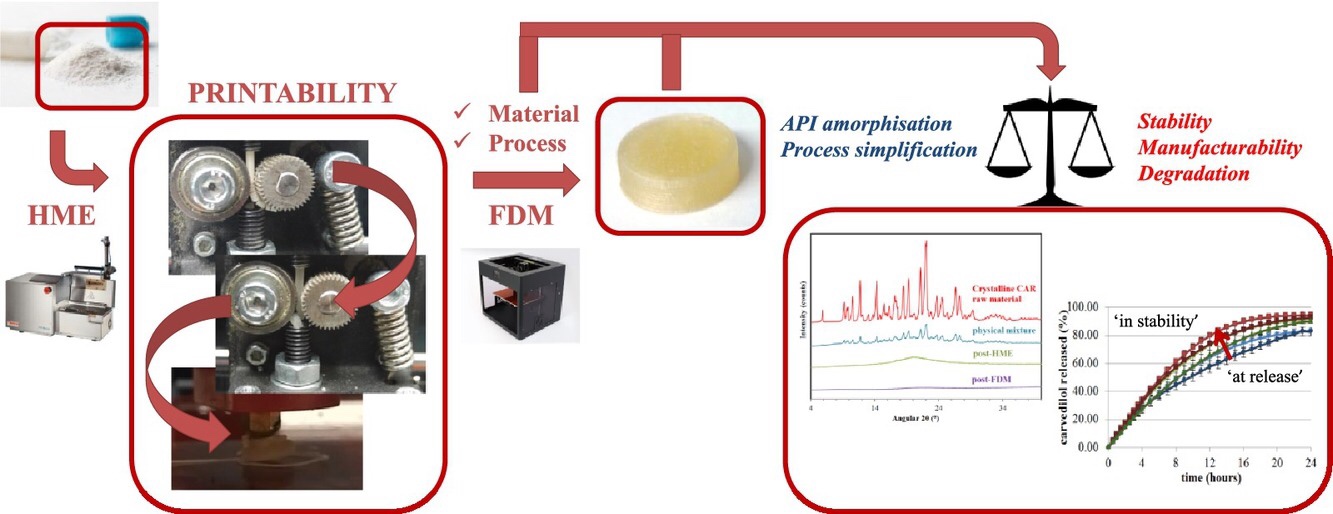
The three dimensional printing (3DP) in the pharmaceutical domain constitutes an alternative, innovative approach compared to the conventional production methods. Fused deposition modelling (FDM), is a simple, cost-effective 3DP technique, however the range of pharmaceutical excipients that can be applied for this methodology is restricted. The study set to define the requirements of the FDM printability, using as technical support custom made, pharmaceutical polymer based filaments and to evaluate if these new dosage forms can live up to the current GMP/GCP quality standards. Formulation rationale was assessed in accordance to the apparatus functionality. Blends were pre-screened based on the processability under the API (carvedilol) thermogravimetric analysis determined critical limit. The technological process implied the use of FDM coupled with hot melt extrusion (HME), while printability was defined by means of thermal, rheological and mechanical measurements. From the pharmaceutical standpoint, the consistency of the in vitro dissolution kinetics was monitored ‘at release’ and ‘in stability’, while the print process impact was evaluated based on the previously determined processability potential. Results showed that FDM printability is multifactorial, with brittleness and melt viscosity as primary limitation factors. The increase in shear-thinning and flexural modulus can enable broader processability intervals, which in turn proved to be essential in limiting degradation product formation. The 3DP tablets released the API in an extended rate, however the temperature and humidity along production and storage should be carefully considered as it may affect the final product quality in time. In conclusion, HME + FDM can be considered as an alternative production methodology, with prospects of applicability in the clinical sector, however for some formulations extensive packaging development will be necessary before confirming their suitability.

