A novel ethyl cellulose-coated sustained release multiple-unit system of tacrolimus
Abstract
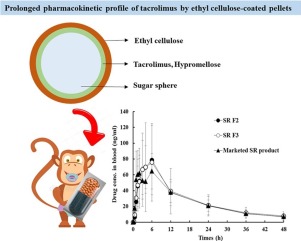
A novel once-a-day sustained-release (SR) system of tacrolimus (FK506), a poorly water-soluble immunosuppressive agent, was designed employing ethyl cellulose (EC) polymer as release retardant. Drug (5 mg) was layered onto sugar spheres (518.3 mg) with hypromellose (5 mg), to transform the drug from a crystalline to an amorphous form. Subsequently, the drug-layered pellets were recoated with EC polymer (0.5-1.5 mg) using a fluid bed granulator. Drug release from the reservoir-type pellets was markedly impeded by the outer EC-based coating layer (EC 1 mg), displaying about 60% of drug release after 8 h, regardless of the acidity of the media. In an in vivo pharmacokinetic study in fasted Cynomolgus monkeys, the drug level in blood was gradually increased over 4.7 h and high drug concentration was maintained until 24 h, with an elimination half-life of 16.6 h. There were no statistical differences between the novel SR pellets and the recently marketed SR capsule (Advagraf®, Astellas Pharma, Japan) in terms of maximum blood concentration, area under the curve, and half-life values, in both fasted and fed states. Therefore, the novel EC-coated pellets are expected to be bioequivalent to the commercial SR capsule, providing a once-daily dosing regimen in patients with allogenic rejection.
Conclusions
A novel multiple-unit reservoir-type SR system of FK506 was constructed by layering the drug with HPMC polymer onto the pellets, and subsequently layering with EC polymer as release controller. Drug layering with HPMC onto the pellets process effective enhanced the thermodynamic solubility of the poorly water-soluble immunosuppressive agent in the inner compart- ment, by transforming the drug crystalline state into amorphous form. Afterward, the drug release from the multiple-unit pellets was effectively controlled by adjusting the amount of EC poly- mer, a water-insoluble and low water-permeable polymer, onto the beads. In an in vivo absorption study in fasted monkeys, the drug concentration in blood was gradually increased (Tmax of about 4.7 h) and then gradually declined by 48 h, with the extended elim- ination T1/2 values of 16.6 h. There were no statistical differences in the pharmacokinetic parameters (AUC, Cmax , Tmax , and T1/2 val- ues) between the novel SR pellets and the marketed product, under both fasted and fed states. We conclude that the novel once-a-day SR pellets employing the cellulose derivative as release retardant might be bioequivalent to the commercial SR capsule in patients with allogenic rejection.


