Application of design of experiment approach for investigating the effect of partially pre-gelatinized starch on critical quality attributes of rapid orally disintegrating tablets
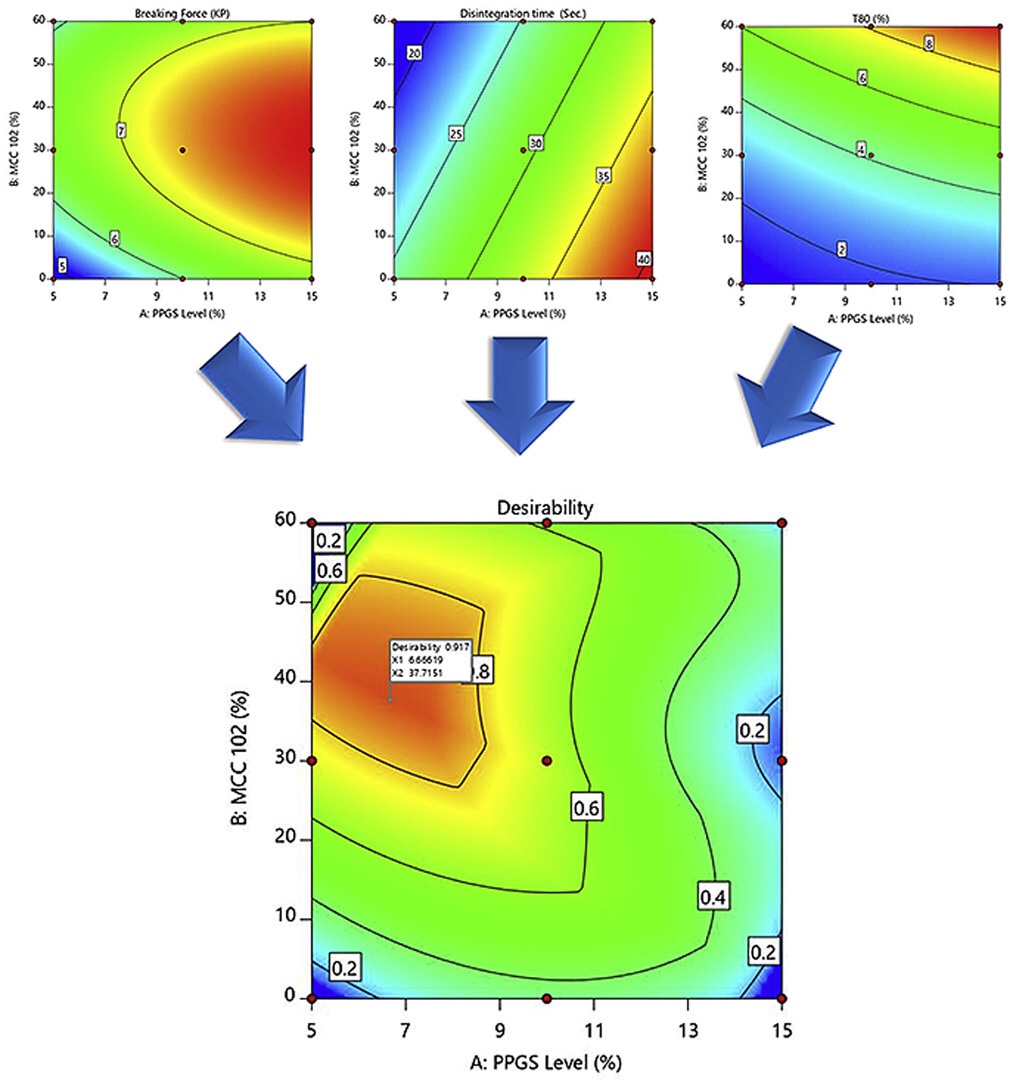
The objective of the current work was to enhance the critical attributes of rapid orally disintegrating tablets (RODTs) manufactured by direct compression technique to have sufficient mechanical strength and fast disintegration time using partially pre-gelatinized starch. A two factor, three levels (32), full factorial design was applied to examine the main and interaction effects of independent variables namely; partially pre-gelatinized starch level (PPGS; X1) and microcrystalline cellulose level (MCC; X2) on the characteristics of RODTs. The produced tablets were tested for their weight uniformity, mechanical strength, disintegration time and in-vitro drug release. Results showed that the manufactured RODTs were compiled with the USP requirements. In addition, ANOVA analysis demonstrated that PPGS level and MCC level had a significant effect (P ≤ 0.05) on the dependent responses. Furthermore, the developed RODTs showed good in-vitro/in-vivo correlation in the disintegration time. Numerical optimization using desirability approach was also applied to optimize the independent variables. It was found that the higher desirability (0.917) could be achieved at low level of PPGS (6.66%) and medium level of MCC (37.71%). Finally, it could be conclude that PPGS is a potential candidate for production of RODTs of high mechanical strength and very fast disintegration time.

