Pharmatrans adds SANAQ® SP205 and SP206 to Direct Compression excipients range
Pharmatrans Sanaq has added SANAQ® SP 205/206 to its DiCom range of Co-Processed Excipients supporting Direct Compression (DC) applications.
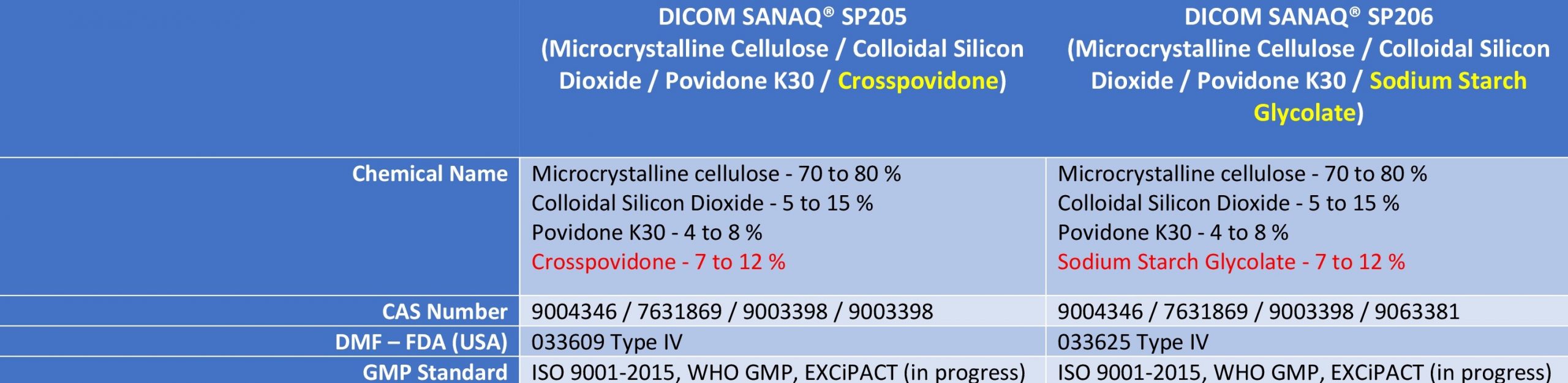
DiCom SANAQ® SP205 and SP206 are both based on microcrystalline cellulose (MCC), co-processed with colloidal silicon dioxide (CSD) and Povidone K30 binders. Whereas SP205 uses Crosspovidone as disintegrant, the SP206 grade prefers Sodium Starch Glycolate (see Table 1).
SANAQ® SP205/SP206 – Features and Benefits
Both excipients have average particle size distribution (PSD) of 300 – 600 microns and are intended for DC processing of hygroscopic and moisture-sensitive active pharmaceutical ingredients (APIs), with the sub micronized CSD fumed silica with average particle size around 15nm providing high surface area (200-400m2/g) that provides a uniform microenvironment for high moisture absorbance and close adherence to API.
In particular, they ppossess excellent and superior flow properties compared to simple physical blends, as measured on all the key parameters such as bulk density, repose angle, Hausner ratio and Carr index.
Protection for sensitive APIs
These are excipients that are tailored and specifically designed for moisture sensitive and low bulk density ‘fluffy’ APIs, with the combination of MCC and CSD providing synergistic protection for fragile ingredients.
While processing of CSD at high concentrations of up to 15% has previously proved problematic, SANAQ’s successful co-processing eases manufacturing, proving advantages of uniform quality and ease of processing.
Table 1:
DiCom SANAQ® SP205 and SP206 specifications compared
see also the DiCom SANAQ® SP205 and SP206 data sheet: Data sheet SANAQ SP 205206


