Dual Drug Loaded Lipid Nanocarrier Formulations for Topical Ocular Applications
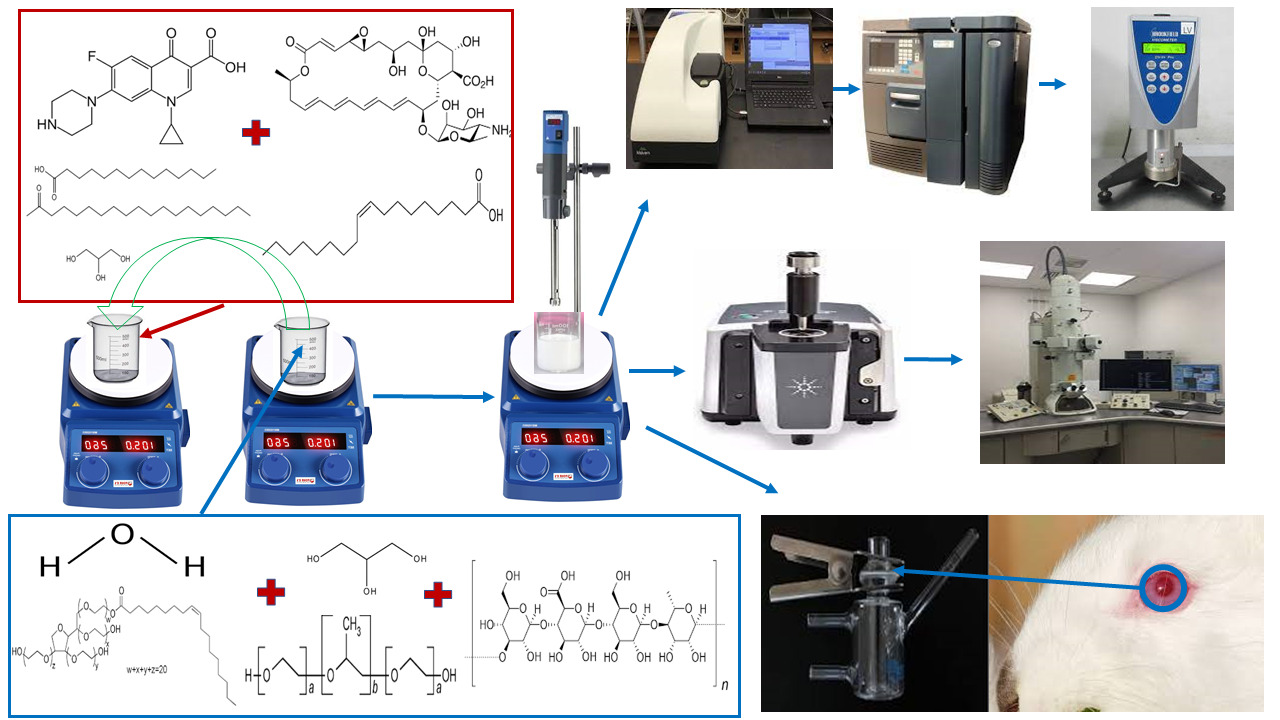
Introduction:
Untreated ocular infections can damage the unique fine structures of the eye with possible visual impairments and blindness. Ciprofloxacin (CIP) ophthalmic solution is prescribed as first-line therapy in ocular bacterial infections. Natamycin (NT) ophthalmic suspension is one of the progenitors in ocular antifungal therapy. Nanostructured lipid carriers (NLCs) have been widely examined for ocular penetration enhancement and distribution to deeper ocular tissues. The objective of the current study was to prepare NLCs loaded with a combination of CIP and NT (CIP-NT-NLCs) and embed them in an in-situ gelling system (CIP-NT-NLCs-IG). This novel formulation will target the co-delivery of CIP and NT for the treatment of mixed ocular infections or as empirical treatment in case of limited access to healthcare diagnostic services.
Methods:
CIP-NT-NLC and CIP-NT-NLC-IG formulations were evaluated based on physicochemical characteristics, in vitro release, and ex vivo transcorneal permeation studies and compared against commercial CIP and NT ophthalmic eye drops.
Results and Discussion:
NLCs formulation (0.1% CIP and 0.3% NT) showed particle size, polydispersity index, and zeta potential of 196.2 ± 1.2 nm, 0.43 ± 0.06, and − 28.1 ± 1.4 mV, respectively. Moreover, CIP-NT-NLCs showed entrapment efficiency of 80.9 ± 2.9 and 98.7 ± 1.9% for CIP and NT, respectively. CIP-NT-NLCs-IGformulation with 0.2% w/v gellan gum demonstrated the most favorable viscoelastic characteristics for ocular application. CIP-NT-NLCs and CIP-NT-NLCs-IG formulations exhibited a sustained release pattern for both drugs over 24 h. Moreover, CIP-NT-NLCs and CIP-NT-NLC-IG formulations showed 4.0- and 2.2-folds, and 5.0- and 2.5-folds enhancement in ex vivo transcorneal permeability of CIP and NT, respectively, compared to the control formulations.
Conclusion:
The results suggest that this dual nanoparticulate-based in-situ gelling drug delivery system can serve as a promising topical delivery platform for the treatment of ocular infections.
Download the full research paper as PDF: Dual Drug Loaded Lipid Nanocarrier Formulations for Topical Ocular Applications
Materials:
NT was purchased from Cayman Chemicals (Ann Arbor, MI, USA). CIP base (CAS number: 85721-33-1) was obtained from Sigma Aldrich (St. Louis, MO). Glyceryl distearate (Precirol® ATO 5) was a generous gift sample from Gattefossé (Paramus, NJ, USA). Poloxamer 188, oleicacid, castor oil, Polysorbate 80, Amicon® Ultra centrifugal filter devices with regenerated cellulose membrane (molecular weight cut off 100 kDa), 0.5 mL cup-like design Thermo Scientific™ Slide-A-Lyzer™ MINI Dialysis Device (10K molecular weight cutoff) and High Performance Liquid Chromatography (HPLC) grade solvents were acquired from Fischer Scientific (Hampton, NH, USA). Gellan gum was obtained from MP Biomedicals, LLC (Santa Ana, CA, USA). Centrifuge tubes, HPLC vials, and scintillation glass vials were obtained from Fischer Scientific (Hampton, NH, USA). The whole eyes of male albino New Zealand rabbits were obtained from Pel-Freez Biologicals (Rogers, AR, USA).
Youssef AAA, Dudhipala N, Majumdar S. Dual Drug Loaded Lipid Nanocarrier Formulations for Topical Ocular Applications. Int J Nanomedicine. 2022;17:2283-2299
https://doi.org/10.2147/IJN.S360740

