Influence of compression kinetics during tableting of fluidized bed-granulated microorganisms on microbiological and physical-mechanical tablet properties
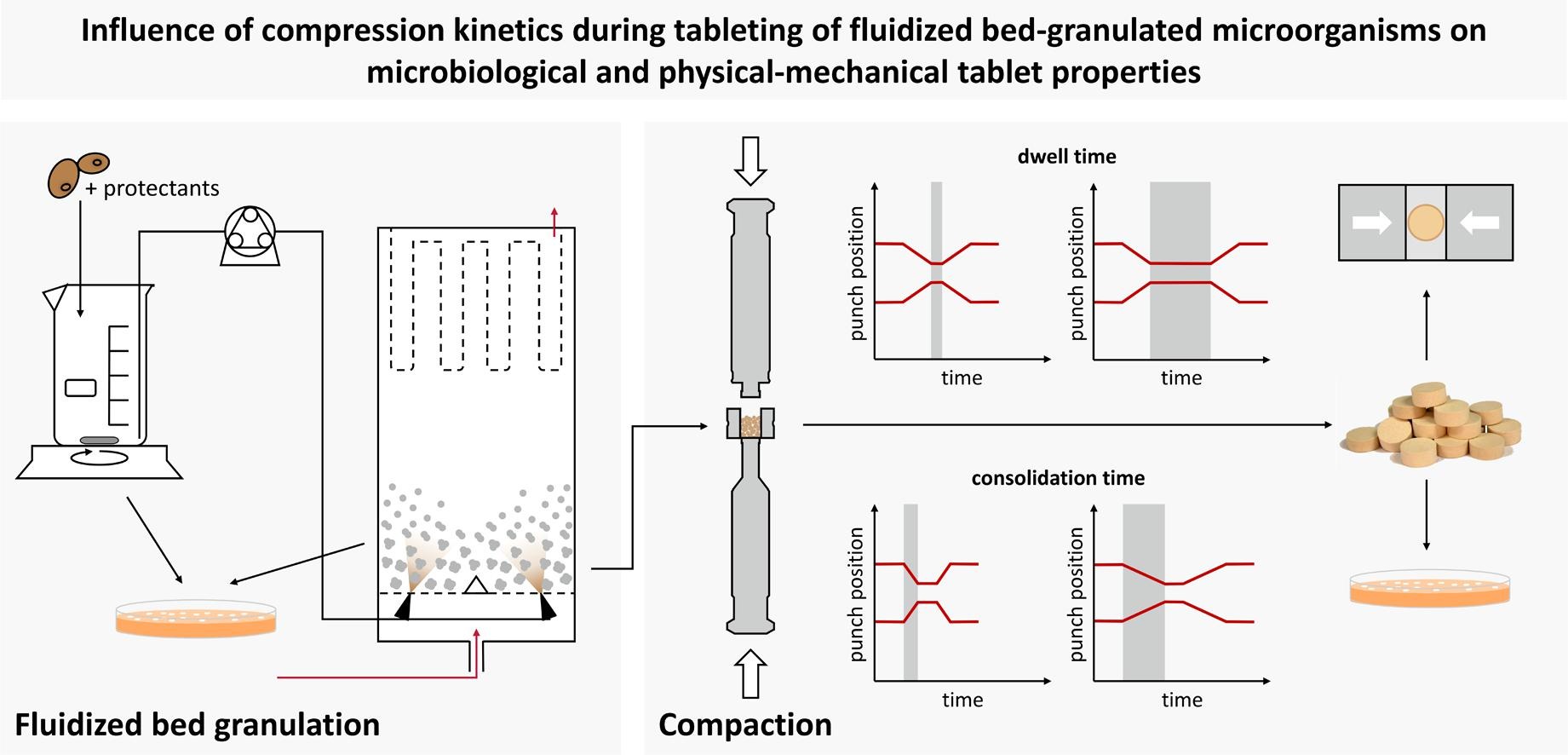
As tablets are convenient to administer to patients, ensure safe dosing and allow cost-effective production on a large scale, they are the favored dosage form for numerous active pharmaceutical ingredients but also for the administration of viable probiotic microorganisms. Granules with viable yeast cells (Saccharomyces cerevisiae) formed by fluidized bed granulation with dicalcium phosphate (DCP), lactose (LAC) or microcrystalline cellulose (MCC) as carrier materials were tableted using a compaction simulator. Besides the compression stress, the compression speed was systematically studied by varying consolidation time and dwell time.
The microbial survival as well as physical properties of the tablets, e.g., porosity and tensile strength, were determined. Higher compression stresses result in lower porosities. While on the one hand this has a detrimental effect on microbial survival (due to increased pressure and shear stress during particle rearrangement / densification), on the other hand it results in higher tensile strengths. At the same compression stress, a prolonged dwell time resulted in lower porosity and thus in lower survival rates but higher tensile strength. Against that, consolidation time showed no significant influence on the considered tablet quality attributes. Since changes of the tensile strength related survival rate were negligible (due to opposite but balancing dependence on porosity), high production speeds could be used for tableting of these granules without additional loss of viability, as long as tablets with the same tensile strength are produced.
Read more on fluidized bed-granulated microorganisms
Karl Vorländer, Lukas Bahlmann, Arno Kwade, Jan Henrik Finke, Ingo Kampen, Influence of compression kinetics during tableting of fluidized bed-granulated microorganisms on microbiological and physical-mechanical tablet properties, European Journal of Pharmaceutics and Biopharmaceutics, Volume 188, 2023, Pages 161-169, ISSN 0939-6411,
https://doi.org/10.1016/j.ejpb.2023.05.012.

