An evaluation of the Johanson model for roller compaction process development for a high dose API
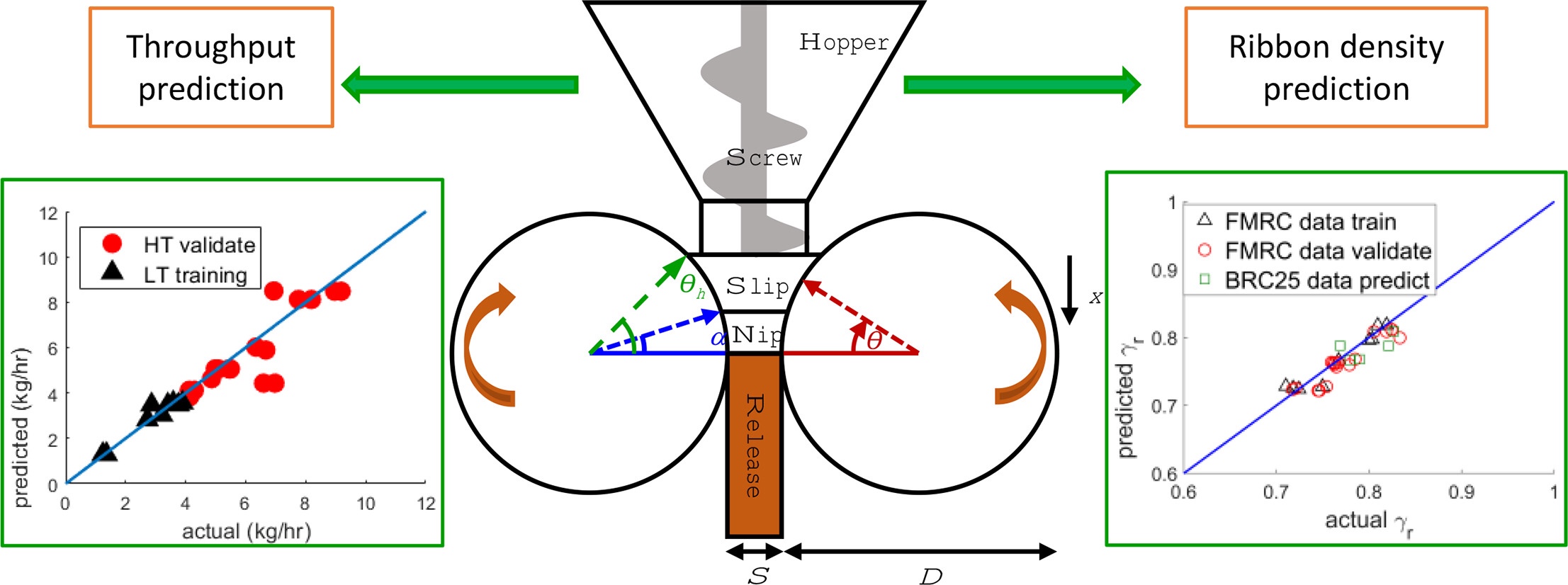
Roller compaction (RC) is a dry granulation technique applied to improve the flow and compressibility of drug formulations. RC implementation for high drug load formulations can be challenging due to flow issues and a high consumption of active pharmaceutical ingredient (API) for robust process development.
This work addresses these challenges using process modelling for design and scale-up of an RC process on the same equipment and transfer to different equipment. A modified application of existing models incorporating a new description of mass transport in the feed screw is evaluated for guaifenesin formulations with a 90% drug loading.
The model is calibrated using low-throughput data on a Vector Freund TF Mini RC and used to predict ribbon density and throughput for various process settings at high-throughput. It is found that the modelling framework can reasonably predict high-throughput behaviour on the same RC but the predictive performance decreases for transfer between equipment. More on roller compaction for high dosed API

