Challenges of Dissolution Methods Development for Soft Gelatin Capsules
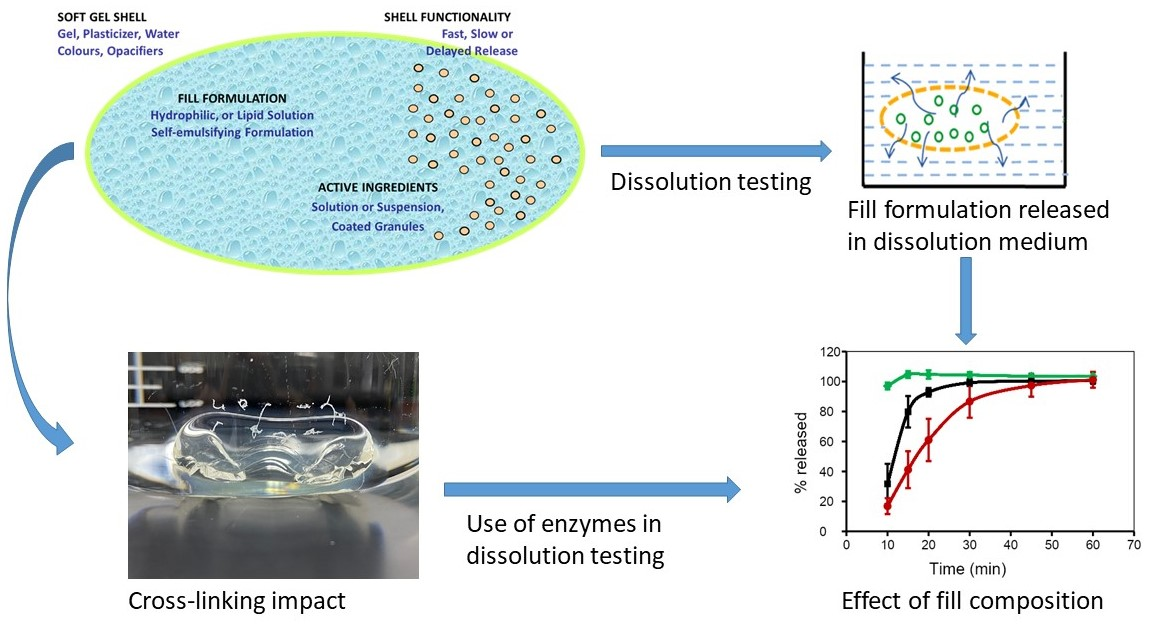
Recently, the development of soft gelatin capsules (SGCs) dosage forms has attracted a great deal of interest in the oral delivery of poorly water-soluble drugs. This is attributed to the increased number of poorly soluble drugs in the pipeline, and hence the challenges of finding innovative ways of developing bioavailable and stable dosage forms. Encapsulation of these drugs into SGCs is one of the approaches that is utilized to deliver the active ingredients to the systemic circulation to overcome certain formulation hurdles.
Once formulated, encapsulated drugs in the form of SGCs require suitable in vitro dissolution test methods to ensure drug product quality and performance. This review focuses on challenges facing dissolution test method development for SGCs. A brief discussion of the physicochemical and formulation factors that affect the dissolution properties of SGCs will be highlighted. Likewise, the influence of cross-linking of gelatin on the dissolution properties of SGCs will also be discussed.
Download the full article here: Challenges of Dissolution Methods Development for Soft Gelatin Capsules
or continue reading here: Damian, F.; Harati, M.; Schwartzenhauer, J.; Van Cauwenberghe, O.; Wettig, S.D. Challenges of Dissolution Methods Development for Soft Gelatin Capsules. Pharmaceutics 2021, 13, 214. https://doi.org/10.3390/pharmaceutics13020214
News on Softgel Caps – Vegetarian Softgel System

Comprising pea starch; carrageenan – a setting agent sourced from marine macro algae; NEOSORB® sorbitol – a plant-based ingredient suitable for nutraceutical application; and Na+ (salt) – a jellifying agent; Roquette’s LYCAGEL™ system offers a plant-based solution suitable for both nutraceutical and pharmaceutical applications that meets EU and US pharmacopeia standards. Continue reading on LYCAGEL™

