Robustness Optimization of an Existing Tablet Coating Process Applying Retrospective Knowledge (rQbD) and Validation
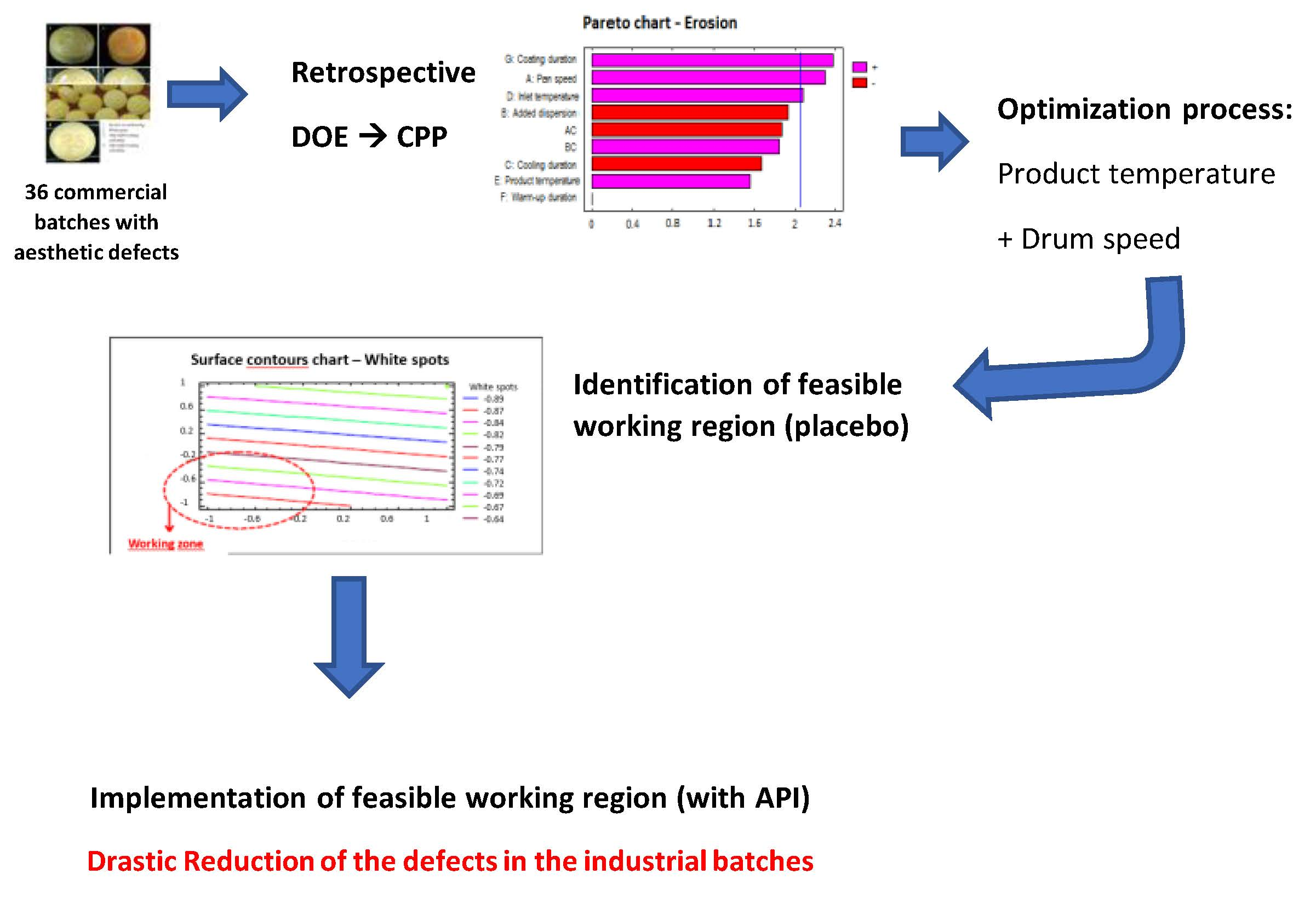
The objective of these studies is to verify and validate the improvement in the inter-tablet coating uniformity for an industrially commercialized coated tablet, without involving changes in the approved registration dossier. Using the CPP (critical process parameters) determined from previous retrospective statistical analysis, the recommended working ranges are identified. Retrospective analysis showed that the design of experiments (DoE) provided an improved process variable configuration. Therefore, it is decided to study two critical parameters: Product temperature and drum speed, with an additional 22 experimental design. The quality results of the samples analyzed show that the aesthetic defects of the batches made with the new working ranges have been reduced. These results have also been corroborated with the 42 industrial batches manufactured with the new ranges. With the optimized parameters, tablets have been coated and the suitability of the model determined. The results demonstrated the overall reliability and effectiveness of the proposed Quality by Design approach and provides a useful tool to help optimize the industrial coating process. This study confirms that it is possible to optimize and validate the manufacturing process of an existing commercial product by means of a DoE with retrospective data. Therefore, no variation in the dossier is required.
Download the full MDPI publication here: Robustness Optimization of an Existing Tablet Coating Process Applying Retrospective Knowledge (rQbD) and Validation
or continue reading here: Galí, A.; Ascaso, M.; Nardi-Ricart, A.; Suñé-Pou, M.; Pérez-Lozano, P.; Suñé-Negre, J.M.; García-Montoya, E. Robustness Optimization of an Existing Tablet Coating Process Applying Retrospective Knowledge (rQbD) and Validation. Pharmaceutics2020, 12, 743.
Keywords: retrospective data; design of experiments; critical process parameters; coating optimization; coating defects; process validation, crospovidone, polysorbate, mannitol, colonial anhydrous silica, microcrystalline cellulose, sodium starch glycolate, hypromellose, magnesium stearate, Opaglos II yellow

