Formulation development of SYN-004 (ribaxamase) oral solid dosage form, a β-lactamase to prevent intravenous antibiotic-associated dysbiosis of the colon
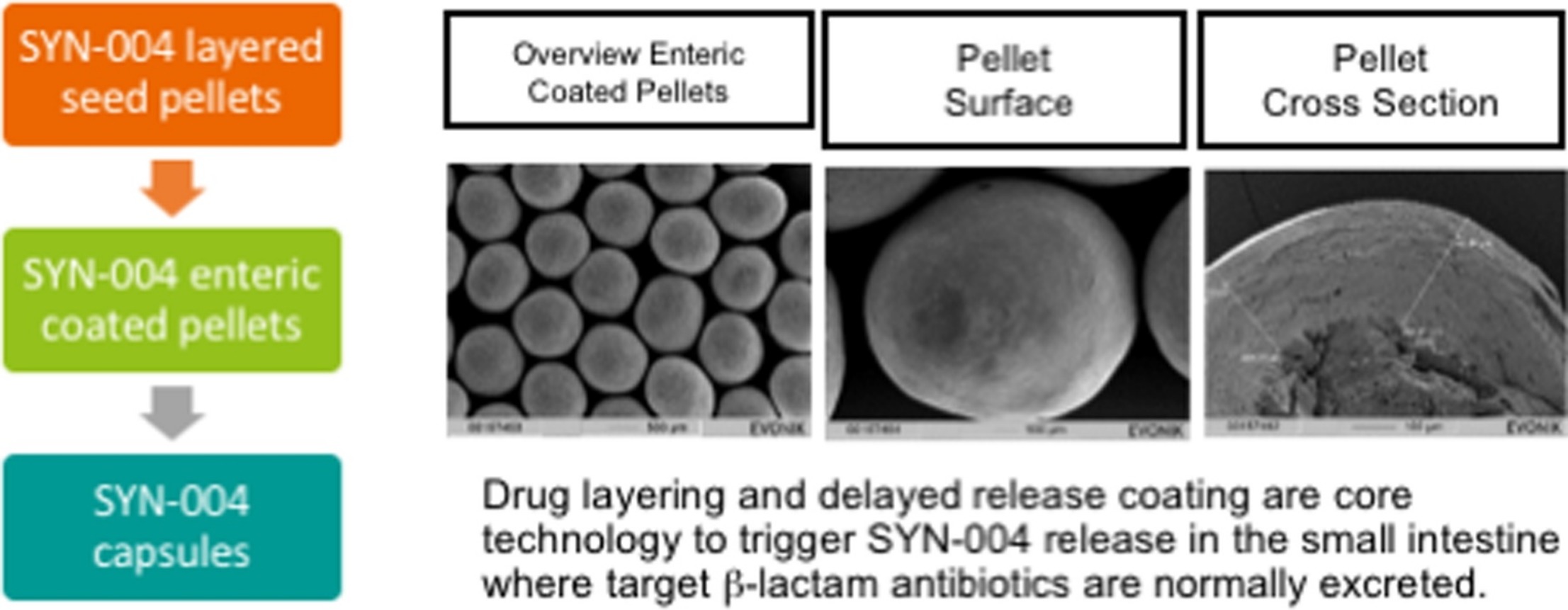
SYN-004 (ribaxamase) delayed release drug product is a multi-particulate, hard capsule for oral delivery of a recombinant β-lactamase enzyme designed to degrade β-lactam antibiotics administered intravenously, and thus prevent colon dysbiosis. Here we describe the development of the SYN-004 enteric coated pellet formulation, which has been tested in multiple clinical trials.
Since the SYN-004 drug substance is a buffered liquid, several binder excipients in different ratios were tested to facilitate binding of SYN-004 to sugar spheres. The binding systems were evaluated by droplet pre-evaluation and film casting tests. The most promising formulations were produced in small scale fluidized bed application runs and analyzed by dissolution tests and complementary analytical assays. Hydroxypropyl cellulose was selected as the preferred SYN-004 binding excipient. The formulation included a second, outer coat containing the enteric EUDRAGIT® L 30 D-55 polymer-based formulation to achieve gastric protection, and rapid SYN-004 release in the intestinal tract, when the pH rises above 5.5. Additional formulation improvements resulted in an increase in the SYN-004 load compared to a predecessor oral enzyme formulation (Ipsat P1A).
Thus, a novel formulation and process for an orally administered enzyme was developed and used to manufacture drug product for clinical trials. More on SYN-004 (ribaxamase) oral solid dosage form, a β-lactamase to prevent intravenous antibiotic-associated dysbiosis of the colon
Download the article here: Formulation development of SYN-004 (ribaxamase) oral solid dosage form, a β-lactamase to prevent intravenous antibiotic-associated dysbiosis of the colon
or continue reading here: Andrew Bristol, Steven Hubert, Felix Hofmann, Hans Baer,
Formulation development of SYN-004 (ribaxamase) oral solid dosage form, a β-lactamase to prevent intravenous antibiotic-associated dysbiosis of the colon, International Journal of Pharmaceutics, Volume 534, Issues 1–2,
2017, Pages 25-34, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2017.10.001.
Excipients
Sucrose sugar spheres (Pharm-a-spheres neutral 600–710) μm, polysorbate-80, glycerin, lactose (Pharmatose® 450, polyvinyl pyrrolidone (PVP) (Kollidon® K12 and K25 hydroxypropyl cellulose), (HPC) (Klucel™ EF Pharm, , Triethyl Citrate (TEC) (CITROFOL® AI, low viscosity hydroxypropyl methylcellulose (HPMC) (Sheffield Biosciences, USA), Coni-Snap® hard gelatin capsules size #0 white/white, methacrylic acid – ethyl acrylate copolymer dispersion 30% (EUDRAGIT® NM 30 D), methacrylic acid – ethyl acrylate copolymer (1:1) dispersion 30% (EUDRAGIT® L 30), glycerol monostearate 40–55% (GMS) (Imwitor® 900K).

