Soluplus® polymeric nanomicelles improve solubility of BCS-class II drugs
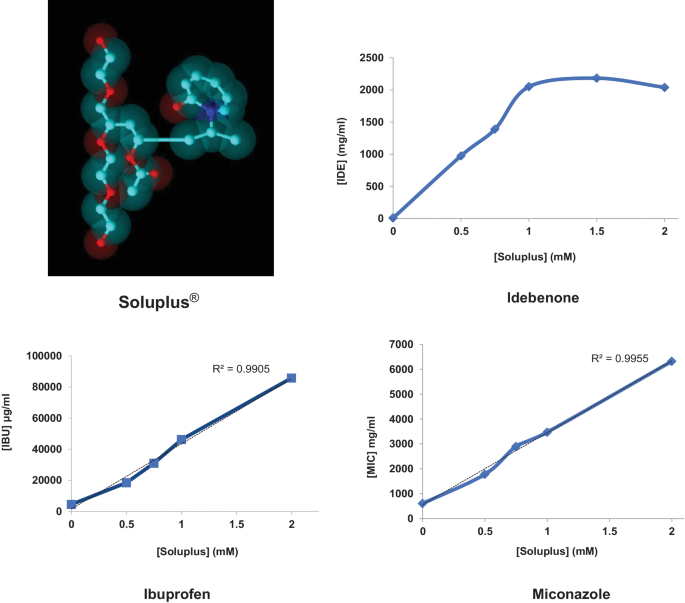
The issue of poor aqueous solubility is often a great hitch in the development of liquid dosage forms for those drugs that the Biopharmaceutics Classification System (BCS) includes in classes II and IV. Among the possible technological solutions, inclusion of the drug molecule within polymeric micelles, and particularly nanomicelles, has been proposed in the last years as a valid strategy. Our attention has been recently attracted by Soluplus®, an amphiphilic polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer able to form small and stable nanomicelles. The aim of this study was to characterize Soluplus® nanomicelles to enhance the apparent solubility of three model APIs, categorized in BCS class II: ibuprofen (IBU), idebenone (IDE), and miconazole (MIC). Drug-loaded Soluplus® micelles with a mean size around 60–70 nm were prepared by two methods (direct dissolution or film hydration method). The prepared nanosystems were characterized in terms of mean particle size and Zeta potential, physical stability, drug solubility, and in vitro drug release. The solubility of the tested APIs was shown to increase linearly with the concentration of graft copolymer. Soluplus® can be easily submitted to membrane filtration (0.2 µm PES or PTFE membranes), showing the potential to be sterilized by this method. Freeze-drying enabled to obtain powder materials that, upon reconstitution with water, maintained the initial micelle size. Finally, viscosity studies indicated that these nanomicelles have potential applications where a bioadhesive material is advantageous, such as in topical ocular administration.
Continue reading here
About this article: Pignatello, R., Corsaro, R., Bonaccorso, A. et al. Soluplus® polymeric nanomicelles improve solubility of BCS-class II drugs. Drug Deliv. and Transl. Res. (2022). https://doi.org/10.1007/s13346-022-01182-x

