Polymeric Amorphous Solid Dispersions of Dasatinib: Formulation and Ecotoxicological Assessment
Abstract
Introduction
Dasatinib (DAS) is a second-generation tyrosine kinase inhibitor used for the treatment of Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) and acute lymphoblastic leukemia [1]. DAS is of particular importance for patients who have developed resistance or intolerance to imatinib, the first tyrosine kinase inhibitor approved for clinical use and used in prior therapy [2,3]. As the disease progresses, resistance to imatinib becomes more common, affecting around 40–66% of patients with advanced-phase CML and 38% of patients with chronic-phase CML. DAS is commercially supplied as Sprycel®—film-coated tablets manufactured by Bristol Myers Squibb and available in various dosage strengths [4]. It falls within class II of the Biopharmaceutical Classification System (BCS), which is characterized by poor aqueous solubility but good intestinal permeability. Its limited solubility in water leads to slow and insufficient absorption, resulting in low oral bioavailability ranging from 14% to 34% [5,6].
Improving the bioavailability of poorly-soluble crystalline drugs such as DAS can be achieved through a promising approach: modifying their crystalline structure with supramolecular changes, such as the preparation of co-crystals, polymorphs, or co-amorphous systems [7]. Usually, the crystalline form of drugs is preferred for stability and purity reasons in formulations [8]. Nevertheless, the amorphous solid state presents advantages, such as improved solubility and release rate, attributable to a reduced energy barrier for dissolution [9]. Yet, amorphous drugs tend to recrystallize during transit, packaging, and storage, posing stability challenges [10]. To effectively address these stability issues, a practical approach involves converting the crystalline drug into its amorphous state and embedding it within an amorphous polymeric matrix, resulting in the formation of an amorphous solid dispersion (ASD). This advanced formulation technique holds great promise for improving the aqueous solubility of poorly water-soluble anticancer drugs such as DAS [11]. The inclusion of a polymeric matrix plays a crucial role in enhancing the physical stability and regulating the drug release rate by inhibiting its conversion into crystalline form, primarily by limiting drug mobility [12]. The preferred intermolecular interactions between the drug and the polymer, such as hydrogen bonds, π–π, or ionic interactions, do not affect the drug’s pharmacological activity but enhance the system’s physical stability. Even in the absence of specific interactions, ASD can remain stable due to molecular-mixing levels [10]. To avoid the thermodegradation of thermosensitive drugs and the use of toxic and hazardous organic solvents, the method of drug mechanochemical activation by co-grinding with amorphous polymers to obtain ASDs is gaining attention as the most environmentally friendly approach [7]. Amorphization achieved through co-grinding results in several favorable outcomes, such as increasing the specific surface area, reducing the particle size of the drug, and enhancing wettability, all of which contribute to an improved drug dissolution rate [13]. Among the frequently used polymers for this purpose, polyvinylpyrrolidone (PVP) stands out due to its hydrophilicity, low toxicity, and biocompatibility [14]. In addition to grinding, which aims to reduce particle size and increase the drug’s surface area in contact with the solvent, various other methods have been employed successfully to improve the release properties of many poorly soluble drugs. These methods include supercritical fluid-based processes [15,16,17], spray drying [18,19,20], lyophilization [21,22,23], electrospinning [24,25,26], and various others.
Numerous methods have been documented to obtain amorphous DAS, with some involving the use of hazardous organic solvents such as dimethylformamide, 1,2-dichlorobenzene, and ethylene glycol [27]. One approach involved preparing solid dispersions of DAS and various polymers through a solvent evaporation method by using water, organic solvents (such as ethanol, methanol, or isopropanol), and hydrochloric acid [28]. Notably, improved drug release was achieved by preparing an ASD of DAS and cellulose acetate butyrate through the solvent evaporation method using acetone [5]. However, residual solvents present a risk of inducing the reversion of amorphous DAS to its crystalline form, altering the drug’s properties. These forms are not stable nor suitable for pharmaceutical development. Consequently, there is a need to establish a process that is both efficient and less hazardous, while also being economically viable and environmentally friendly, to address two of the big challenges that the pharmaceutical industry faces nowadays: the overuse of hazardous organic solvents and the formulation of drugs with low solubility, such as DAS. One promising solution is mechanochemical activation by grinding, which is a fast and simple method that not only meets economic and environmental standards, but also overcomes issues related to solubility, solvent complexation, or solvolysis [29]. For example, co-grinding DAS with hydroxypropylmethylcellulose acetate succinate and Kollidon® VA 64 was described in US patent 9,249,134 B2 [30].
As a result of DAS’s poor solubility in human intestinal fluids (HIFs), another significant ecological issue arises. Namely, anticancer drugs often undergo limited metabolism within the body, leading to their excretion unchanged through urine and feces by patients [31]. This process poses an environmental concern, as these drugs are continuously released into the aquatic environment, causing acute and chronic toxicity to both the aquatic ecosystem and human health [32,33]. The environmental monitoring of DAS’s trace-level residues in wastewater and biological samples has been documented in the literature in concentrations up to 150 µg L−1 [34]. However, to date, no studies have been published on the ecotoxicity of DAS resulting from its accumulation in the environment.
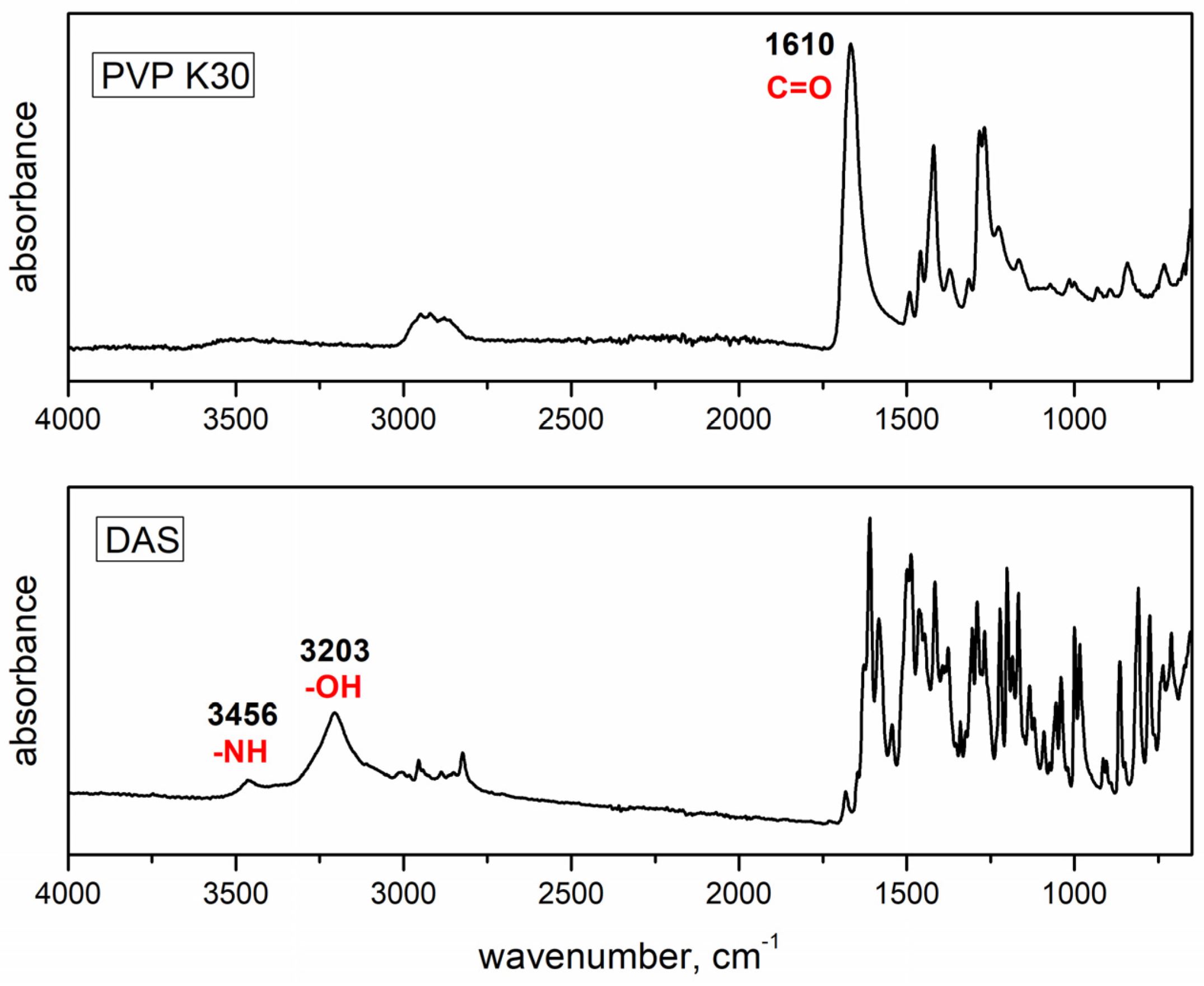
The main objective of this study was to prepare ASDs of DAS within a PVP K30 matrix using an environmentally friendly co-grinding method. Hopefully, such a formulation will enhance the release rate of DAS. Different parameters for co-grinding were chosen—such as grinding jars and balls made of materials of different hardnesses, as well as different revolution speeds—to investigate the effect on the drug amorphization rate. The ASDs obtained through this process were thoroughly characterized using several techniques: differential scanning calorimetry (DSC) for thermal analysis, Fourier transform infrared spectroscopy (FTIR) for the investigation of possible hydrogen bonding between DAS and PVP, and X-ray powder diffraction (XRPD) for detection of the amorphization level of DAS as a result of co-grinding with PVP. These methods can confirm the successful preparation of ASDs and predict the enhancement of the drug release rate. Subsequently, in vitro dissolution of the drug from obtained ASDs was tested and compared to that of the untreated drug, providing valuable insights into the effectiveness of the co-grinding approach in enhancing drug release. To investigate the ecotoxicological effects of the native form of the drug, the following test organisms were selected: Vibrio fischeri, a marine bacterium; Pseudomonas putida, a saprophytic bacterium; Chlorella sp., a freshwater microalga; and Lemna minor, a floating duckweed. The detailed investigation of ecotoxicological effects on these selected organisms not only contributes to our understanding of the drug’s environmental impact but also informs strategies for sustainable drug development. This conscientious examination aligns with contemporary principles of ecological risk assessment, emphasizing the importance of evaluating pharmaceuticals in a broader environmental context.
Download the full article as PDF here Polymeric Amorphous Solid Dispersions of Dasatinib
or read it here
Materials
Dasatinib monohydrate was generously supplied by Teva Pharmaceutical Industries (Zagreb, Croatia). Polyvinylpyrrolidone with an average molecular weight of 30 kDa was purchased from Acros Organics (Newark, NJ, USA). Sodium acetate anhydrous was obtained from Lach-Ner (Neratovice, Czech Republic). Triton X-100 was purchased from Biochem Chemopharma (Cosne-Cours-sur-Loire, France), and glacial acetic acid was purchased from Alkaloid AD Skopje (Skopje, North Macedonia). Chlorella sp. and Lemna minor were obtained from the Department of Zoology at the University of Zagreb, Faculty of Science, Zagreb, Croatia.
Sokač, K.; Miloloža, M.; Kučić Grgić, D.; Žižek, K. Polymeric Amorphous Solid Dispersions of Dasatinib: Formulation and Ecotoxicological Assessment. Pharmaceutics 2024, 16, 551. https://doi.org/10.3390/pharmaceutics16040551
Read more articles on “Polymeric Amorphous Solid Dispersions“ here:
- Effects of Additives on the Physical Stability and Dissolution of Polymeric Amorphous Solid Dispersions
- Advances in the Development of Amorphous Solid Dispersions
- Solubility improvement of curcumin by crystallization inhibition from polymeric surfactants in amorphous solid dispersions