Paediatric clinical study of 3D printed personalised medicines for rare metabolic disorders
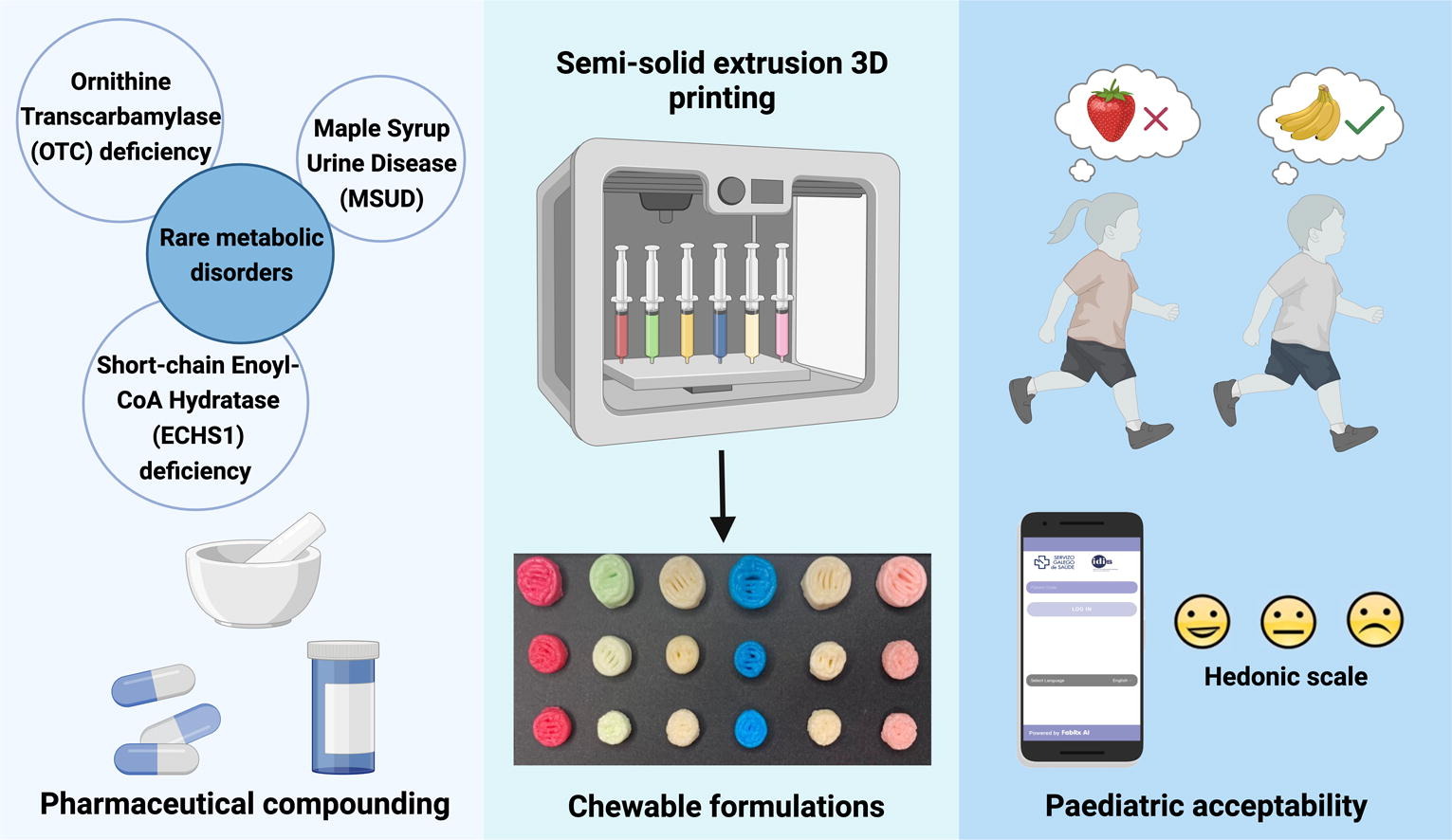
Rare diseases are infrequent, but together they affect up to 6–10 % of the world’s population, mainly children. Patients require precise doses and strict adherence to avoid metabolic or cardiac failure in some cases, which cannot be addressed in a reliable way using pharmaceutical compounding. 3D printing (3DP) is a disruptive technology that allows the real-time personalization of the dose and the modulation of the dosage form to adapt the medicine to the therapeutic needs of each patient.
3D printed chewable medicines containing amino acids (citrulline, isoleucine, valine, and isoleucine and valine combinations) were prepared in a hospital setting, and the efficacy and acceptability were evaluated in comparison to conventional compounded medicines in six children. The inclusion of new flavours (lemon, vanilla and peach) to obtain more information on patient preferences and the implementation of a mobile app to obtain patient feedback in real-time was also used. The 3D printed medicines controlled amino acid levels within target levels as well as the conventional medicines. The deviation of citrulline levels was narrower and closer within the target concentration with the chewable formulations.
According to participants’ responses, the chewable formulations were well accepted and can improve adherence and quality of life. For the first time, 3DP enabled two actives to be combined in the same formulation, reducing the number of administrations. This study demonstrated the benefits of preparing 3D printed personalized treatments for children diagnosed with rare metabolic disorders using a novel technology in the real clinical practice over the current approach.
Download the full article as PDF here Paediatric clinical study of 3D printed personalised medicines for rare metabolic disorders
or read it here
Materials
L-Isoleucine (ILE) (Nutricia, Utrecht, The Netherlands), L-Valine (VAL) (Nutricia, Utrecht, The Netherlands) and L-Citrulline (CIT) (Nutrición Médica, Cantabria Labs, Madrid, Spain) were used as the active ingredients for both the conventional and 3D printed chewable medications. Conventional medication consisted of hard gelatine capsules (Acofarma, Barcelona, Spain) and Avicel® PH 102 microcrystalline cellulose (Acofarma, Barcelona, Spain). Chewable formulation was property of FABRX Ltd. (London, UK). Yellow, green, blue bright and red colorants were purchased in Guinama, (Valencia, Spain). Red colorant for pink colour was purchased in Acofarma (Barcelona, Spain). Strawberry, banana, orange, lemon, peach and vanilla flavours were purchased in Acofarma (Barcelona, Spain).
Lucía Rodríguez-Pombo, María José de Castro-López, Paula Sánchez-Pintos, Jose Maria Giraldez-Montero, Patricija Januskaite, Goretti Duran-Piñeiro, M. Dolores Bóveda, Carmen Alvarez-Lorenzo, Abdul W. Basit, Alvaro Goyanes, Maria L. Couce, Paediatric clinical study of 3D printed personalised medicines for rare metabolic disorders, International Journal of Pharmaceutics, 2024, 124140, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.124140.
Read also our introduction article on 3D Printing here:


