Development of direct compression Acetazolamide tablet with improved bioavailability in healthy human volunteers enabled by cocrystallization with p-Aminobenzoic acid
Abstract
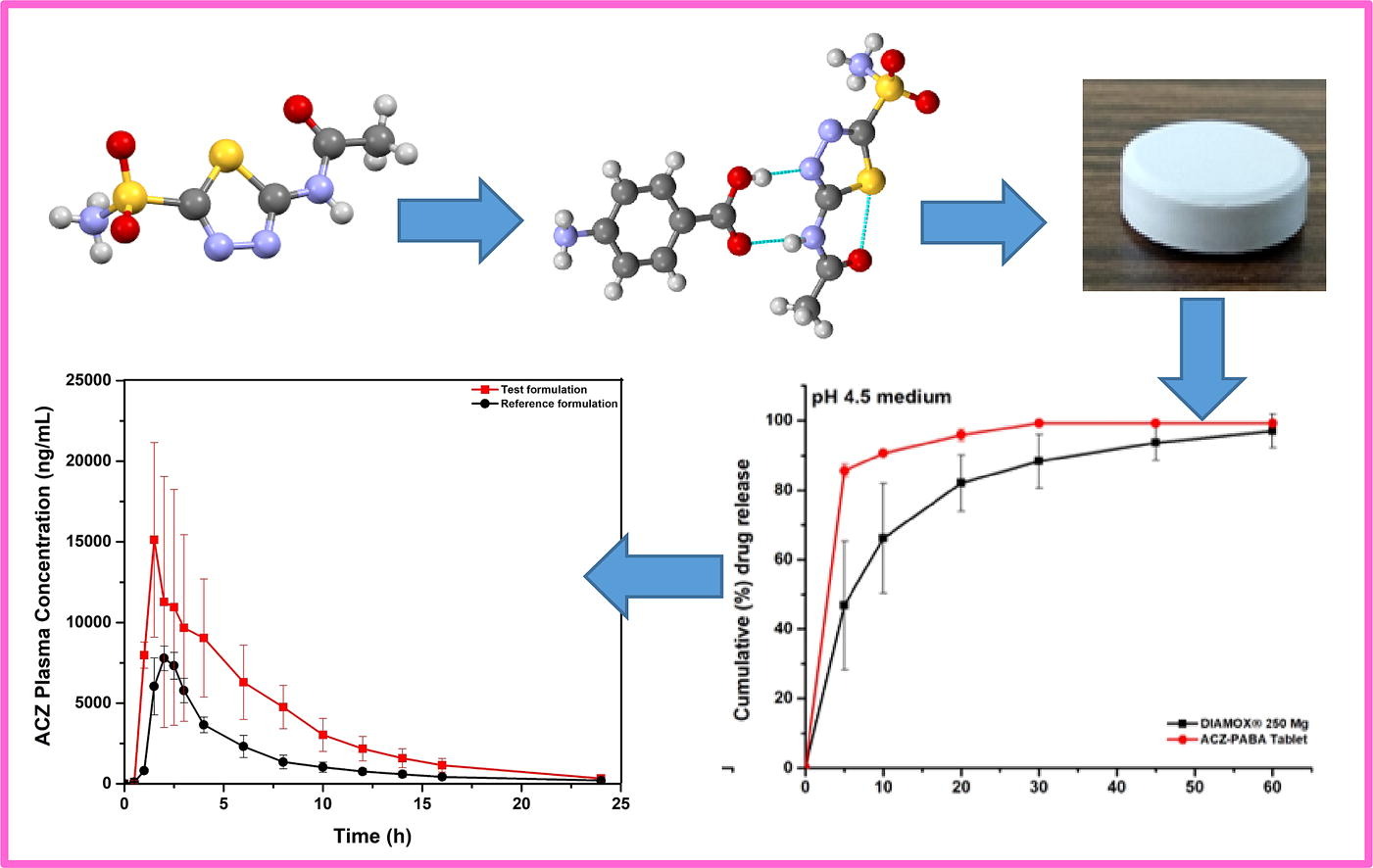
Pharmaceutical cocrystallization has been widely used to improve physicochemical properties of APIs. However, developing cocrystal formulation with proven clinical success remains scarce. Successful translation of a cocrystal to suitable dosage forms requires simultaneously improvement of several deficient physicochemical properties over the parent API, without deteriorating other properties critical for successful product development. In the present work, we report the successful development of a direct compression tablet product of acetazolamide (ACZ), using a 1:1 cocrystal of acetazolamide with p-aminobenzoic acid (ACZ-PABA). The ACZ-PABA tablet exhibits superior biopharmaceutical performance against the commercial tablet, DIAMOX® (250 mg), in healthy human volunteers, leading to more than 50% reduction in the required dose.
Introduction
Acetazolamide, 5-acetamido-1,3,4-thiadiazole-2-sulfonamide (ACZ), is a carbonic anhydrase inhibitor primarily used to treat glaucoma (Arenas-García et al., 2010, Fang et al., 2021) It is also used as an adjuvant therapy for epilepsy, diuresis, and high-altitude sickness, oedema caused by congestive heart failure, and preventing drug-related side effects in the treatment of influenza (Arenas-García et al., 2012) Given the many therapeutic benefits, World Health Organization (WHO) has included ACZ in the list of essential medicines.(Meers et al., 2020) However, being a Biopharmaceutical Classification System (BCS) class IV drug, ACZ suffers from both low solubility and low permeability (Pandey and Ghosh, 2022, Zhang et al., 2019). In addition, poor flowability and tabletability of ACZ affect its formulation development.(Di Martino et al., 2001, Kaur et al., 2002) To overcome these challenges, researchers have attempted various formulation strategies, including nanoparticles(Verma et al., 2013), microspheres (Haznedar and Dortunç, 2004), liposomes (El-Gazayerly and Hikal, 1997), inclusion complexes (Gladys E. Granero et al., 2008), spray drying(Di Martino et al., 2001) and cocrystals (Arenas-García et al., 2010, Manin et al., 2020, Meers et al., 2020). Over the years, pharmaceutical cocrystals have emerged as an important tool to improve the poor physicochemical properties of active pharmaceutical ingredients (APIs) without compromising their pharmacological activities.(Ji et al., 2022, Kale et al., 2017, Kumari et al., 2019, Wong et al., 2021, Yousef and Vangala, 2019) A main strength of cocrystallization lies in its wide range of applicability to improve physicochemical properties of APIs, thus providing an array of benefits at the same time.(Chow et al., 2012) Consequently, cocrystals have gained considerable interest from the pharmaceutical industry, academic institutions, and drug regulatory bodies across the world (Aitipamula et al., 2012, Kumari and Ghosh, 2020, Roy and Ghosh, 2020).
ACZ consists of a sulfonamide group, an acetamide and a thiadiazole group (Figure 1), which provides potential sites for non-covalent interactions with complementary coformers. Thus, many cocrystals of ACZ with various acidic and amide coformers were reported in literature, which are mostly focused on the preparative strategies and structural determination (Arenas-García et al., 2012, Arenas-García et al., 2010, Bolla and Nangia, 2016, Kakkar et al., 2018), with limited evaluation of their physicochemical properties (Manin et al., 2020, Song et al., 2019, Zhang et al., 2019). Moreover, a majority of the reported coformers were not in the Generally Regarded As Safe (GRAS) list, making them less attractive for tablet formulation development. Furthermore, due to the poor flowability and tabletability properties of ACZ, tablets are usually prepared using a resource-intensive wet granulation process instead of the more efficient direct compression process (Duggirala et al., 2016, Huang, 2004, Karki et al., 2009, Schultheiss and Newman, 2009). Because these stated problems can be addressed by modifying crystal packing (Aitipamula and Vangala, 2017, Sun, 2009), cocrystallization would provide an opportunity to simultaneously improve these physicochemical properties to enable successful pharmaceutical tablet development (Arenas-García et al., 2017). In this context, it is also of great significance to explore the applicability of cocrystals of ACZ in improving the biopharmaceutical properties. In this work, we systematically evaluated the cocrystal of ACZ with p-amino benzoic acid (PABA), which could be prepared by both mechanochemical and slurry-based sonication assisted method (Manin et al., 2020). The ACZ-PABA cocrystal demonstrates improved aqueous solubility in comparison to ACZ and, importantly, PABA is a GRAS listed coformer. In the present work, bulk ACZ-PABA cocrystal was prepared using simplified slurry-based techniques and physicochemical properties, such as permeability and tabletability, were studied. The improved tabletability of the ACZ-PABA led to a successful development of an ACZ tablet by direct compression, which also exhibited an excellent safety profile and comparative pharmacokinetic profiles in healthy human volunteers.Fig 2.
Read more here
Materials
Acetazolamide, 99.8% was obtained as a gift from Nakoda Chemicals Ltd. (Hyderabad, India). PABA, 99% was purchased from Sigma-Aldrich (St. Louis, Missouri, USA). Microcrystalline cellulose (MCC, AVICEL® PH101) and Croscarmellose sodium (CCS, AC-DI-SOL®) was purchased from Signet Excipients Private Limited (Mumbai, India). Fumed silica (CAB-O-SIL® M-5P) was purchased from Cabot Sanmar Limited (New Delhi, India). All other inactive ingredients were of pharmaceutical grade.
Nimmy Kumari, Parag Roy, Sukanta Roy, Chenguang Wang, Sourav Das, Noopur Pandey, Susanta Kumar Mondal, Anirbandeep Bose, Changquan Calvin Sun, Animesh Ghosh, Development of direct compression Acetazolamide tablet with improved bioavailability in healthy human volunteers enabled by cocrystallization with p-Aminobenzoic acid, International Journal of Pharmaceutics, 2024, 123793, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.123793.
Read more on Disintegrants here:


