Chlorogenic acid-optimized nanophytovesicles: a novel approach for enhanced permeability and oral bioavailability
Background
Chlorogenic acid, a phenolic derivative, shows excellent pharmacological properties. However, poor lipidic solubility, permeability, and oral bioavailability restrict its clinical use. Therefore, two different phospholipids—Phospholipon® 90H and LIPOID® S100 nanophytovesicles (NPVs)—were optimized, formulated and compared with central composite design for improved biopharmaceutical properties, antioxidant, anticancer and wound-healing activities.
Results
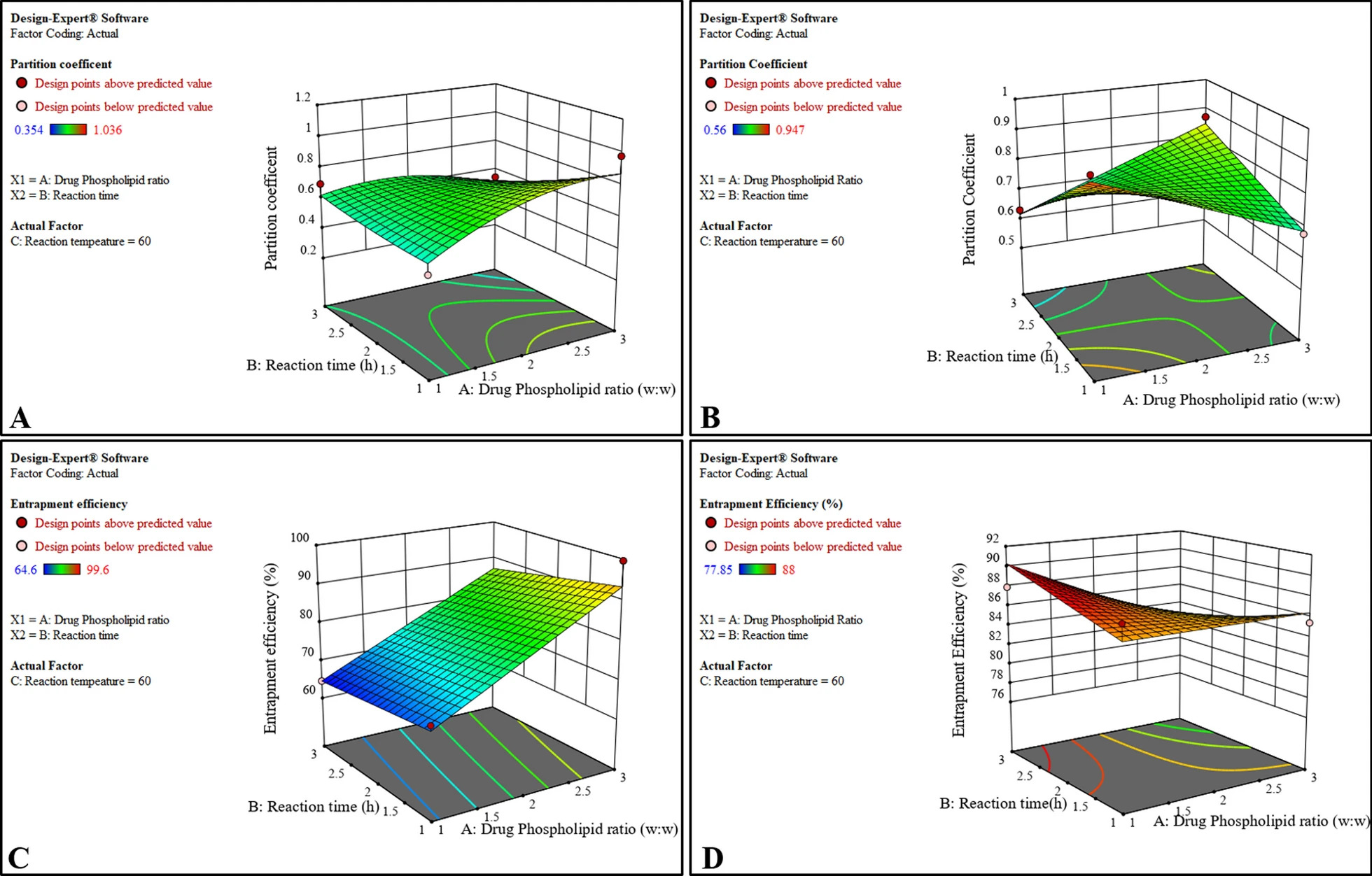
Higher entrapment (> 95%) and partition coefficient values were obtained with optimized CGA 90H NPVs and S100 NPVs. Particle size and zeta potential values confirmed small particle size(≅ 450 nm) with optimum stability. Non-covalent interactions between CGA and both phospholipids were confirmed with Fourier transform infrared spectrophotometry, differential scanning calorimetry and proton nuclear magnetic resonance. NPVs significantly enhanced the lipidic solubility (> 25 times) supported by high-performance thin-layer chromatography. A sustained dissolution and diffusion release were obtained with NPVs as compared to pure CGA. Likewise, ≅ twofold increase in permeability was obtained, supported by confocal microscopy. Enhanced oral bioavailability of CGA with improved Cmax, Tmax, AUC, half-life values was obtained with NPVs along with IVIV correlation. Enhanced DPPH radical scavenging and Fe2+ chelation ability were obtained with CGA 90H NPVs > CGA S100 NPVs, with lower IC50 values in HeLa and HL-60 cell lines (< 0.75 times) as compared to CGA in MTT(3-(4,5-dimethylthiazol-2-yl)- 2,5- diphenyltetrazolium bromide) assay. Higher wound contraction percentages were observed at day 3 with CGA S100 NPVs (71.56%) > CGA 90H NPVs (34.0%) in wound-healing studies.
Conclusions
The formulated NPVs exhibited efficiency of Phospholipon®90 H in enhancing oral bioavailability and LIPOID® S100 in increasing transdermal permeability, thus proving as promising carriers for enhancing biopharmaceutical and pharmacological properties of chlorogenic acid.
Materials
CGA was obtained from Chemsworth, India. Soyabean phospholipid LIPOID® S100 and hydrogenated phospholipid Phospholipon® 90H were obtained as gratis samples from Lipoid, Germany. DPPH (2-diphenyl-1-picrylhydrazyl), ferrozine and ferric chloride were obtained from SD fine chemicals, India. Dichloromethane and N-hexane were purchased from Merck, India. Rhodamine 6G and sinapic acid (Internal standard) D7927 were procured from Sigma-Aldrich, USA. Silverex® heal hydrogel (1% colloid silver) (Sun pharmaceuticals In. Ltd.) was purchased from local pharmacy shop. MTT(3-(4,5-dimethylthiazol-2-yl)- 2,5- diphenyltetrazolium bromide) was purchased from Thermo Fisher scientific, India. MEM (Minimum Essential Medium Eagle) and RPMI-1640 (Roswell Park Memorial Institute) medium, foetal bovine serum (FBS) were obtained from HiMedia Lab, India. While all other reagents used in the study were of analytical grade. HL-60 (human leukaemia cell line) and HeLa (Henrietta Lacks cervical cancer cell line) were obtained from National Centre for cell lines, India.
Download the full study as PDF here: Chlorogenic acid-optimized nanophytovesicles: a novel approach for enhanced permeability and oral bioavailability
or read it here
Trivedi, H.R., Puranik, P.K. Chlorogenic acid-optimized nanophytovesicles: a novel approach for enhanced permeability and oral bioavailability. Futur J Pharm Sci 9, 116 (2023).
https://doi.org/10.1186/s43094-023-00559-0

