Significant Progress in Improving Atorvastatin Dissolution Rate: Physicochemical Characterization and Stability Assessment of Self-Dispersible Atorvastatin/Tween 80® Nanocrystals Formulated through Wet Milling and Freeze-Drying
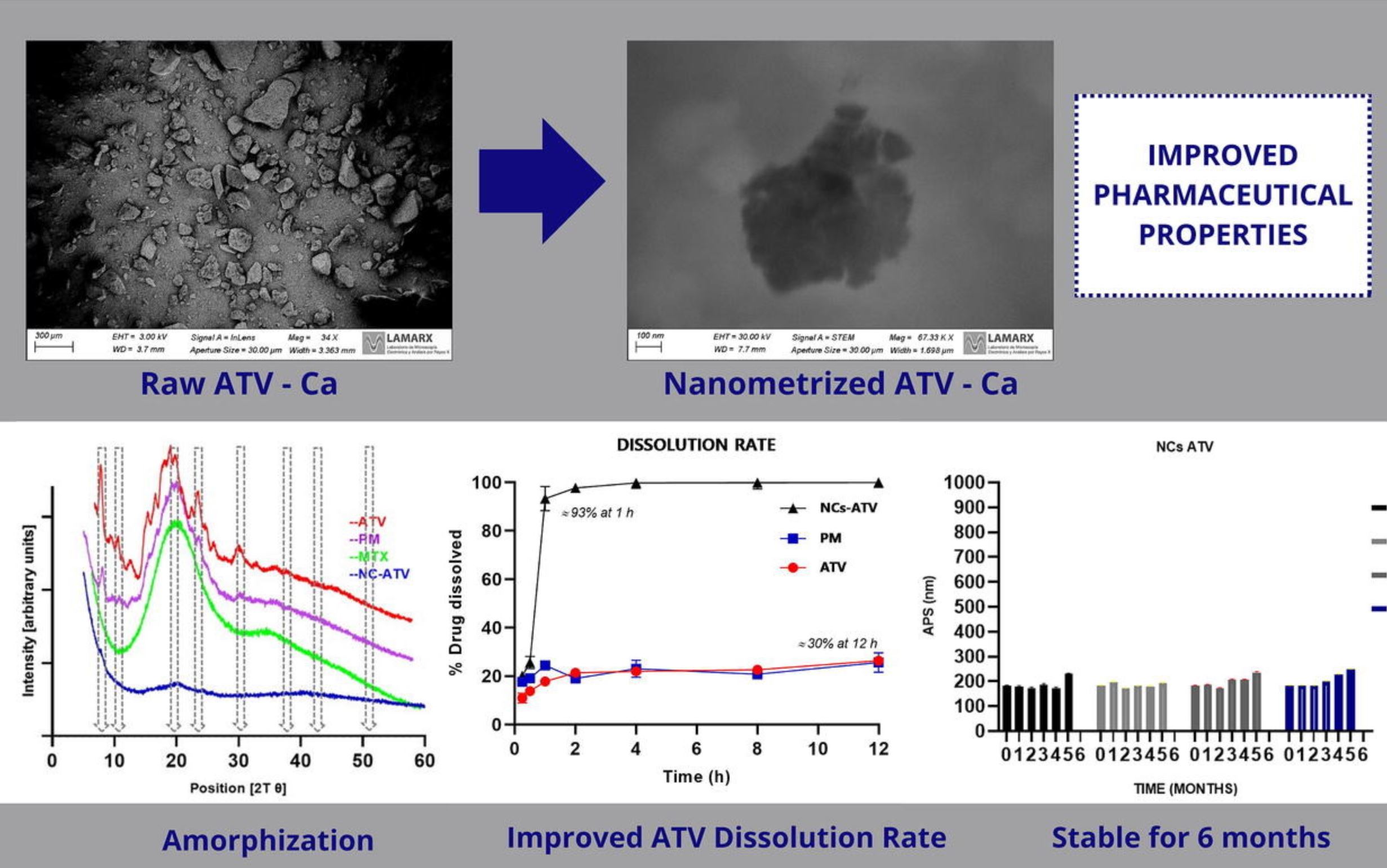
Atorvastatin (ATV) is a first-line drug for the treatment of hyperlipidemia. This drug presents biopharmaceutical problems, partly due to its low solubility and dissolution rate. In this work, nanocrystals of ATV stabilized with Tween 80® were designed by wet milling. A full factorial design was applied to optimize the process. Additionally, a cryoprotectant agent (maltodextrin, MTX) was identified, which allowed maintaining the properties of the nanocrystals after lyophilization. The storage stability of the nanocrystals was demonstrated for six months in different conditions. The obtained nanocrystal powder was characterized using SEM, EDXS, TEM, DSC, TGA, FT-IR, and XRD, showing the presence of irregular crystals with semi-amorphous characteristics, likely due to the particle collision process.
Based on the reduction in particle size and the decrease in drug crystallinity, a significant increase in water and phosphate buffer (pH 6.8) solubility by 4 and 6 times, respectively, was observed. On the other hand, a noticeable increase in the dissolution rate was observed, with 90% of the drug dissolved within 60 minutes of study, compared to 30% of the drug dissolved within 12 hours in the case of the untreated drug or the physical mixture of components. Based on these results, it can be concluded that the nano-milling of Atorvastatin stabilized with Tween 80® is a promising strategy for developing new formulations with improved biopharmaceutical properties of this widely used drug.
Read more here
Materials
Calcium ATV was purchased from the Novalquim S.R.L laboratory (Rosario, Argentina), T80 (Kolliphor PS 80) donated by Rumapel S.R.L (Bs.As, Argentina), Maltodextrin (DE-15)(MTX) purchased from Nicco S.R.L (Cordoba, Argentina).
Alan Rossetti, Daniel Andrés Real, Bruno Andrés Barrientos, Daniel Alberto Allemandi, Alejandro J. Paredes, Juan Pablo Real, Santiago Daniel Palma, Significant Progress in Improving Atorvastatin Dissolution Rate: Physicochemical Characterization and Stability Assessment of Self-Dispersible Atorvastatin/Tween 80® Nanocrystals Formulated through Wet Milling and Freeze-Drying, International Journal of Pharmaceutics, 2023, 123720, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123720.
Read more on “Orally Disintegrating Tablets (ODTs)” here:


