BASF Pharma Solutions excipient accepted into FDA Pilot Program for novel excipients – Press Release

Florham Park, New Jersey, December 5, 2022 –
BASF Pharma Solutions, a global business unit of BASF, announces today that the U.S. Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER), Office of New Drugs, has accepted their excipient, Soluplus®, into the FDA’s Pilot Program for the Review of Innovation and Modernization of Excipients (PRIME). The program seeks to reduce the risk and burden for pharmaceutical companies wishing to utilize novel excipients for modern drug development challenges as well as to provide a pathway for excipient manufacturers like BASF to obtain FDA review of their novel excipients prior to use in an FDA-approved drug. The Soluplus® branded excipient has now moved into the next stage of the extensive evaluation process, and the FDA will share the results at the conclusion of this process.
“With our long history of developing novel excipients, it is especially rewarding that BASF is selected to participate in this pilot program,” states Jeff DeAlmeida, Senior Vice President, BASF Pharma Solutions. “We chose to submit this particular excipient for the FDA’s consideration as it can be used in the development of a wide range of vital medicines.”
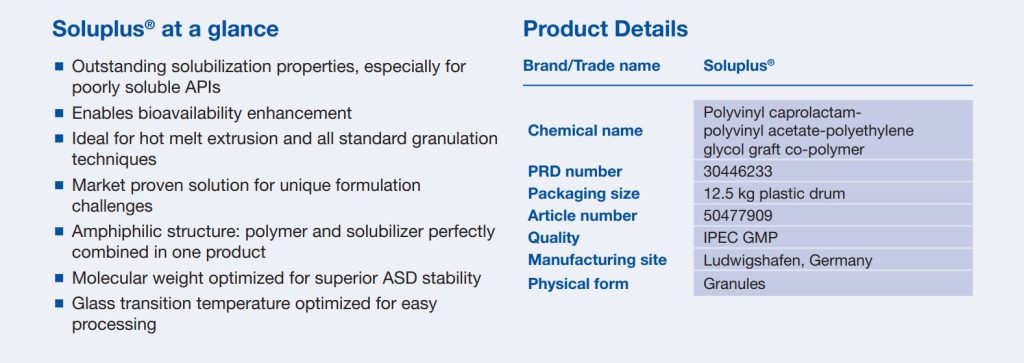
The Soluplus® branded excipient (polyvinyl caprolactam-polyvinyl acetate polyethylene glycol graft copolymer) was specifically designed to improve the solubility and oral bioavailability of poorly soluble active pharmaceutical ingredients, which has been an ever-increasing challenge to the industry. “The review of novel excipients is a critical step in driving innovation in new materials to meet the global health challenges of today and the future,” states Dominik Odenbach, Director of Global Quality, Regulatory Compliance and External Affairs, BASF Pharma Solutions. Soluplus® is particularly suitable as a matrix polymer in hot melt extrusion, a key enabling technology utilized in numerous modern medicines.
“We would like to thank the FDA for introducing the novel excipient Pilot Program. IPEC-Americas, the IQ Pharma Consortium, and the USP all recognize the need for excipient innovation and the challenges for the adoption of novel excipients in drug development. The collaborative efforts among these groups have supported and advocated for this initiative. As the need for novel excipients has never been greater, we are optimistic that a successful pilot program will lead to a formal novel excipient review program in the near future,” says Nigel Langley, Global Technology Director at BASF and a Chair of IPEC-Americas.
BASF is a leader in innovation and has pioneered the development of novel excipients over several decades.
Download the full Press Release here BASF Pharma Solutions excipient accepted into FDA Pilot Program for novel excipients – Press Release
(or click on the picture)
Source: BASF Press Release “BASF Pharma Solutions excipient accepted into FDA Pilot Program for novel excipients”
Soluplus® – For better solubility and bioavailability
Did you know that Soluplus also solubilizes drugs processed by wet granulation?
Try Soluplus® and experience a new dimension in solubility and bioavailability enhancement.
Regulatory Documentation
- US-DMF #23504 (Type IV DMF, containing CMC information)
- Regulatory Information File (RIF) with equivalent content to an Open Part/Applicants Part of a DMF
- US-DMF #23626 (Type V DMF, containing pre-clinical safety data)
For a Letter of Authorization or a copy of the RIF please contact your sales representative.
Preclinical Safety Data
- Tox Abstract (Summary of the design and results of the pre-clinical studies performed)
- Safety Expert Report (Detailed description of the pre-clinical safety data, as well as two clinical study reports.
Available under BASF secrecy agreement)
For a copy of the Tox Abstract, Safety Expert Report and our secrecy agreement please contact your sales
representative.
Pharmacopoeia Monographs and Titles
Soluplus® is not yet monographed in any pharmacopoeia. Based on the approval in several EU member states, BASF
has initiated the application of a monograph in the European Pharmacopoeia. A USP-NF monograph will follow upon
approval in the USA.
Read more on “Soluplus® – For better solubility and bioavailability“
(or click on the picture)
Source: BASF “Soluplus® – For better solubility and bioavailability”




