Design of a Novel Delivery Efficiency Feedback System for Biphasic Dissolving Microarray Patches Based on Poly(Lactic Acid) and Moisture-Indicating Silica
Abstract
Dissolving microarray patches (DMAPs) represent an innovative approach to minimally invasive transdermal drug delivery, demonstrating efficacy in delivering both small and large therapeutic molecules. However, concerns raised in end-user surveys have hindered their commercialization efforts. One prevalent issue highlighted in these surveys is the lack of clear indicators for successful patch insertion and removal time. To address this challenge, a color-change-based feedback system is devised, which confirms the insertion and dissolution of DMAPs, aiming to mitigate the aforementioned problems. The approach combines hydrophilic needles containing model drugs (fluorescein sodium and fluorescein isothiocyanate (FITC)-dextran) with a hydrophobic poly(lactic acid) baseplate infused with moisture-sensitive silica gel particles. The successful insertion and subsequent complete dissolution of the needle shaft are indicated by the progressive color change of crystal violet encapsulated in the silica. Notably, distinct color alterations on the baseplate, observed 30 min and 1 h after insertion for FITC-dextran and fluorescein sodium DMAPs respectively, signal the full dissolution of the needles, confirming the complete cargo delivery and enabling timely patch removal. This innovative feedback system offers a practical solution for addressing end-user concerns and may significantly contribute to the successful commercialization of DMAPs by providing a visualized drug delivery method.
Introduction
Microarray patch (MAP) technology for transdermal drug delivery has expanded exponentially in recent times. During the early stages of development, MAP technology saw considerable success in the cosmetic industry, however, through both academic and industrial collaboration, a wide range of small and large therapeutic molecules have now been successfully delivered across the skin using MAPs.[1, 4] Encouragingly, both patients and healthcare advisors have responded positively to MAP technology. For example, in a recent Phase I clinical trial, 98.6% of participants receiving an inactivated influenza vaccine through a dissolving MAP (DMAP) reported an overall positive experience.[1] This is somewhat unsurprising given the pain-free, minimally invasive nature of DMAP vaccine administration, in contrast to a conventional hypodermic needle and syringe. Furthermore, MAPs can be self-applied, only requiring thumb pressure to achieve successful skin insertion.[2, 3] Removing the need for administration by a healthcare professional has the potential to increase patient acceptance and improve vaccine coverage, particularly in low-resource countries.[4]
Nevertheless, end-user input remains vitally important to the commercial success of DMAP technology. Regulatory authorities have also offered valuable contributions to the field and have proposed requirements that should be considered before DMAPs can be accepted for clinical use. One such requirement is based on the ease and reliability of DMAP application to the skin. Indeed, several concept studies have developed feedback mechanisms as an indirect method of confirming successful skin insertion. This has included the use of a pressure-indicating sensor film,[5] a water-filled reservoir,[6] and snap-based devices.[7] Interestingly, to date, no all-in-one feedback mechanism has the ability to both confirm the successful insertion of the DMAP and indicate when the DMAP should be removed.
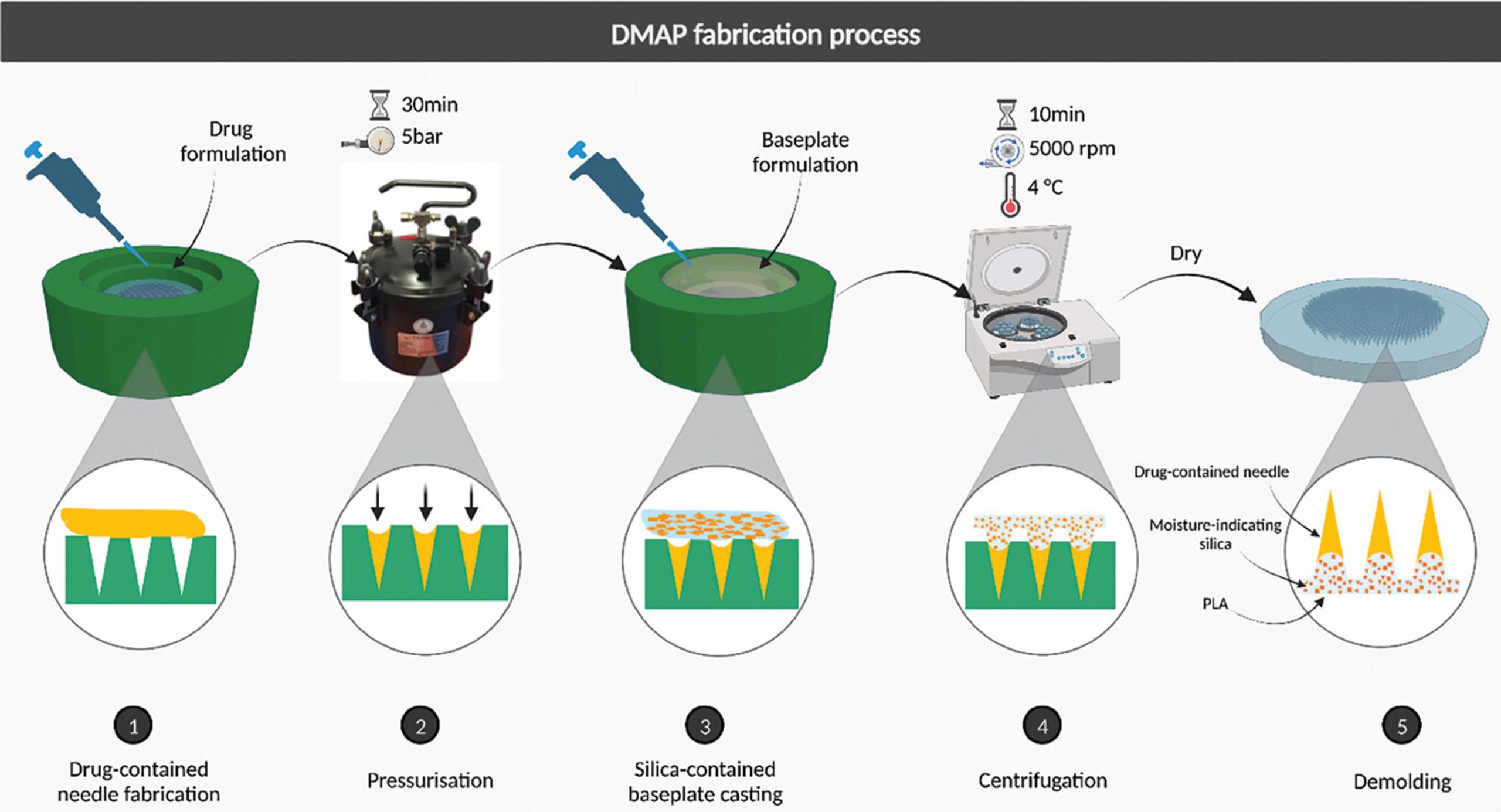
Therefore, in this study, a color-change-based system has been first designed and reported for the purpose of confirming DMAP insertion and dissolution. This has been achieved by incorporating a poly(lactic acid) (PLA) baseplate containing crystal violet dye encapsulated within silica into a DMAP. As a biopolymer, PLA is biodegradable and biocompatible, which is commonly used within the pharmaceutical industry. In addition, dye encapsulation using silica macro particles was selected as a viable method to retain crystal violet whilst preventing its skin absorption. The main principle behind this novel feedback mechanism, however, is based upon the color change of crystal violet when exposed to an aqueous media. This results in a color change from yellow to violet on the surface of the DMAP, producing an absorbance maximum of 590 nm and an extinction coefficient of 87 000 m−1 cm−1. As successful insertion of the DMAP system into the skin results in exposure to interstitial fluid, hydration of the polymeric needles and the baseplate of the DMAP permit the color change of the dye. Not only would this provide a direct method of visibly confirming skin insertion, but the color change intensity could also relate to the dissolution of the drug-loaded needles. This would indicate that the delivery cargo has been deposited successfully into the skin, addressing potential issues with drug dosing from regulatory authorities, healthcare providers, and the end-user. The applicability of this feedback system on the delivery of both small and large therapeutic molecules such as nucleic acids and vaccines through DMAPs has been proved using two separate model compounds, fluorescein sodium (376 Da) and fluorescein isothiocyanate (FITC)-dextran (150 kDa).[8] Furthermore, cytotoxicity studies have been performed to illustrate the biocompatibility of this novel system.
This study has specifically discussed the prominent concerns highlighted by patients, healthcare professionals, and regulatory authorities in using DMAPs and, for the first time, provided innovative solutions, seeking to address the drawbacks associated with the current methods of drug administration. This certainly has imperative implications for the future commercialization of MAP technology.
Download the full article as PDF here: Design of a Novel Delivery Efficiency Feedback System for Biphasic Dissolving Microarray Patches Based on Poly(Lactic Acid) and Moisture-Indicating Silica
or read more here
Materials
PLA Ingeo Biopolymer 3D850 was purchased from NatureWorks (Minnesota, United States). Orange indicator silica pellets were obtained from Interra Global Corporation (Park Ridge, US). FITC, (3-aminopropyl) triethoxysilane (APTES), DCM, PBS tablets (pH 7.3–7.5), poly(vinyl alcohol) (PVA) (9–10 kDa) and FITC-dextran (150 kDa) were purchased from Sigma Aldrich (St. Louis, MO, USA). Fluorescein sodium was purchased from Fluka (Buchs, Switzerland). PVP Plasdone K-29/32 (58 kDa) was obtained from Ashland (Kidderminster, UK). Ultrapure water was obtained from a water purification system (Elga PURELAB DV 25, Veolia Water Systems, Dublin, Ireland).
Huanhuan Li, Qonita Kurnia Anjani, Aaron R. J. Hutton, Juan Luis Paris, Natalia Moreno-Castellanos, Achmad Himawan, Eneko Larrañeta, Ryan F. Donnelly, Design of a Novel Delivery Efficiency Feedback System for Biphasic Dissolving Microarray Patches Based on Poly(Lactic Acid) and Moisture-Indicating Silica, Advanced Healthcare Materials, First published: 12 March 2024 https://onlinelibrary.wiley.com/doi/10.1002/adhm.202304082
See our next webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


