State of the Art in Capsule-Based Dry Powder Inhalers: Deagglomeration Techniques and the Consequences for Formulation Aerosolization
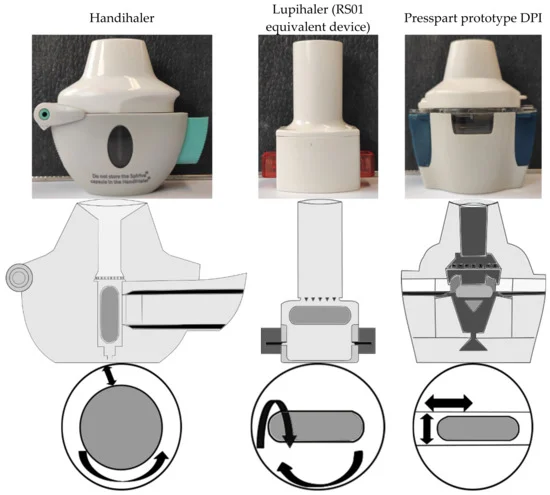
Commercially available dry powder inhalers (DPIs) are usually devices in a fixed combination with the intended formulation, and a change in medication by the physician often forces the patient to use a different device, requiring the patient to relearn how to use it, resulting in lower adherence and inadequate therapy. To investigate whether DPIs can achieve successful outcomes regardless of the formulation and flow rate used, a novel DPI and two commercially available devices were compared in vitro for their deagglomeration behavior for different binary blends and a spray-dried particle formulation. The results demonstrate that the novel device achieved the highest fine particle fraction (FPF) regardless of the formulations tested. In the binary mixtures tested, the highest emitted fraction was obtained by shaking out the powder due to the oscillating motion of the capsule in the novel device during actuation. For DPIs with high intrinsic resistance to airflow, similar FPFs were obtained with the respective DPI and formulation, regardless of the applied flow rate. Additionally, the development and use of binary blends of spray-dried APIs and carrier particles may result in high FPF and overcome disadvantages of spray-dried particles, such as high powder retention in the capsule.
Download the full research paper as PDF: State of the Art in Capsule-Based Dry Powder Inhalers
Groß, R.; Berkenfeld, K.; Schulte, C.; Ebert, A.; Sule, S.; Sule, A.; Lamprecht, A. State of the Art in Capsule-Based Dry Powder Inhalers: Deagglomeration Techniques and the Consequences for Formulation Aerosolization. Pharmaceutics 2022, 14, 1185. https://doi.org/10.3390/pharmaceutics14061185

