The influence of directly compressible fillers/diluents on selected compact properties and drug dissolution: a case study of rivaroxaban
Introduction
Direct compression represents a favorable tablet manufacturing method. However, obtaining satisfactory mechanical properties of the compacts and drug dissolution remains a challenge in formulation development, particularly in the case of challenging model drugs. Rivaroxaban is classified as a class II model drug according to the iopharmaceutical Classification System and exhibits prominent cohesiveness and low aqueous solubility (Choi et al., 2022). The aim of this work was to evaluate the influence of different directly compressible fillers/diluents on selected compact properties (namely, tensile strength, friability, and disintegration) and the dissolution of rivaroxaban from the prepared compacts.
Мaterials and methods
Nine directly compressible fillers/diluents were investigated, namely: Disintequik™ ODT (DQ, Kerry), Ludiflash® (LF, BASF), Parteck® ODT (PO, Merck), CombiLac® (CL, Meggle Pharma), StarLac® (SL, Meggle Pharma), Prosolv SMCC® 90 (PS, JRS Pharma), Klucel™ HF (KC, Ashland), Benecel™ K100M (BC, Ashland), and RetaLac® (RL, Meggle Pharma). Rivaroxaban (RIV) was used as a model drug. Crospovidone (CP, Polyplasdone® XL-10, GAF Chemicals Corporation) was added as a superdisintegrant and magnesium-stearate as a lubricant (1%). The powder mixture composition is represented in Table 1.
Table 1. Sample composition
Powder mixtures were compressed by a single-punch laboratory tablet press (GTP series D, Gamlen Tableting Ltd) with a 6-mm flat punch using a compression load of 400 kg, i.e. 140 MPa. The obtained compacts (100 mg) were analyzed regarding compact thickness, diameter, and hardness (Erweka TBH 125D, Erweka), which were used for tensile strength calculations. Friability was measured using 10 compacts after 4 minutes of rotation (Erweka AR 400, Erweka), at 25 rpm. Disintegration time was investigated using a disintegration tester (Erweka ZT52, Erweka) without discs, with purified water at 37 °C. The dissolution test was performed with a rotating paddle apparatus (Erweka DT 600, Erweka), with a rotation speed of 75 rpm, using a medium consisting of 900 ml of acetate buffer (pH 4.5) with 0.2% sodium-lauryl sulphate.
Results and discussion
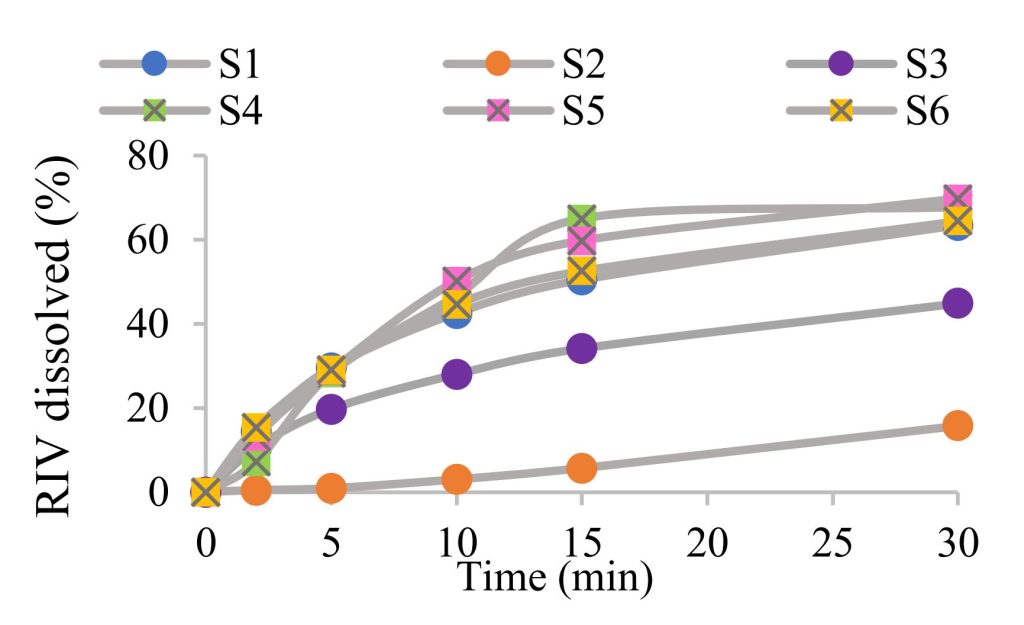
The obtained compacts exhibited high tensile strength values between 1.69 MPa (SL-based S5) and 6.64 MPa (PS-based S6), since a tensile strength higher than 1.7 MPa is acceptable for compact handling and further processing. 4, S5, and S9 compacts exhibited low tensile strength due to a high concentration of poorly compactible lactose monohydrate (e.g., 85% in SL), analogously to the reports by Dominik et al. (2021). The highest tensile strength was observed in the case of S6 compacts due to microcrystalline cellulose in the PS composition (p<0.01). The compact friability was, generally, lower than 1%, as suggested by the European Pharmacopoeia (2.9.7), apart from. The S1 and S5 compacts (friability 1.43% and 1.24%, respectively). With regards to disintegration time, all of the investigated compacts exhibited very fast disintegration, shorter than 30 s (11.5-24.2 s), with the exception of S2 (disintegration time 30.6 min) (p<0.01). A long disintegration time was not expected since LF is intended for fast disintegration tablet formulations, and pure LF compacts disintegrated within 7.31 min. In the case of the investigated immediate release formulations, approximately 65% of RIV dissolved within 30 minutes (Fig. 1). Incomplete dissolution was associated with low RIV solubility. Samples S2 and S3, containing mannitol-based fillers, exhibited slower dissolution and a lower percentage of RIV dissolved after 30 minutes (p<0.001).
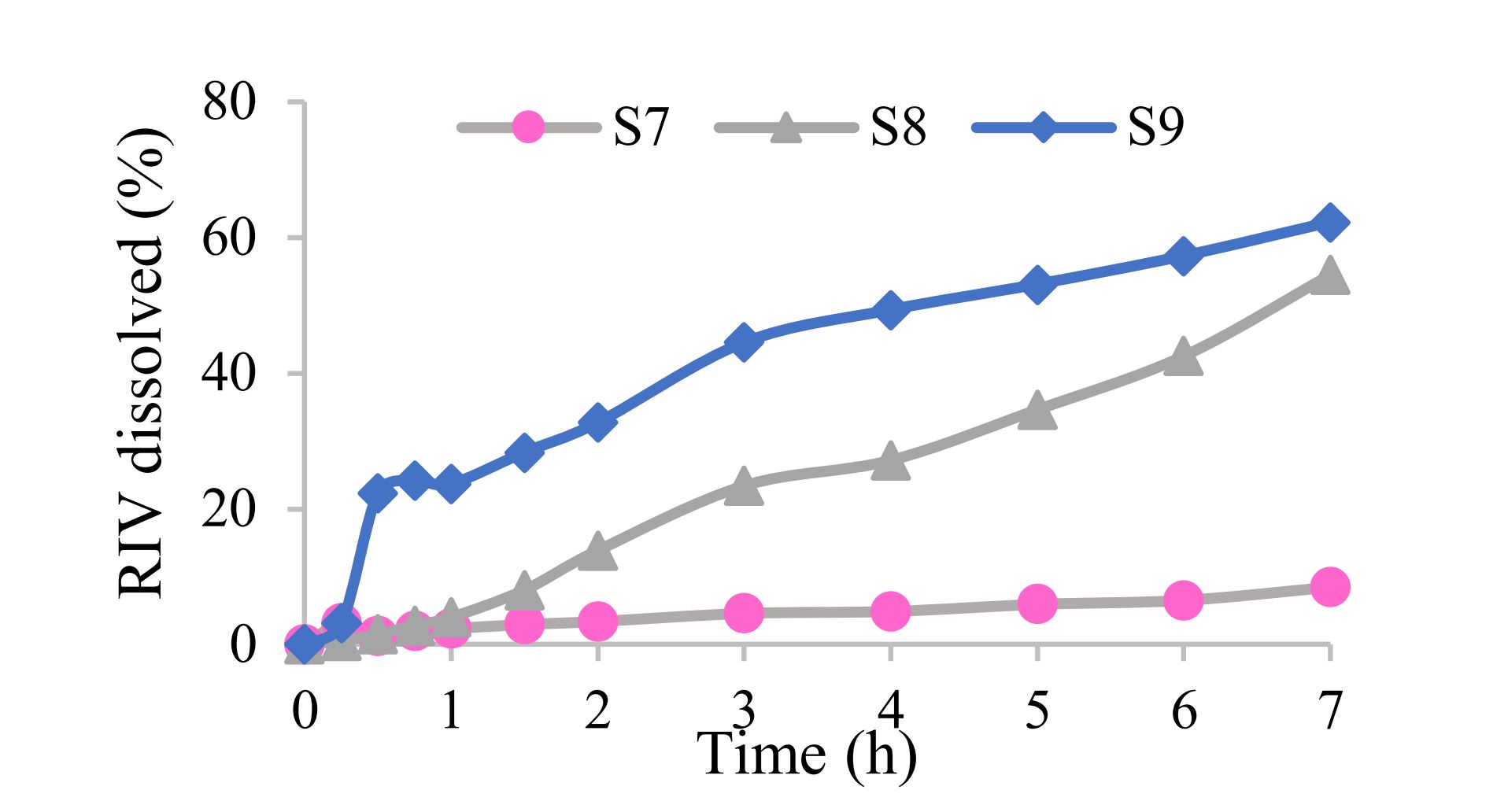
In the case of modified-release compacts, the dissolution profiles were notably affected by the incorporated filler/diluent (Fig. 2). In the case of S7 containing KC (KC molecular weight 1,150,000), the dissolution profile was very slow, resulting in less than 10% RIV dissolved after 7 hours. The manufacturer suggests 20–30% KC in the modified release tablets, so a suitable low molecular-weight filler/diluent should be added. Both BC (molecular weight 1,000,000) and RL are based on hypromellose type 2208. The RIV dissolution was faster from the S9 due to composition of RL, which contains lactose, and the lower hypromellose concentration in the compact (p<0.001).
See the full brochure on “A case study of Rivaroxaban” here
(click the picture to download the brochure)
Source: PDF “A case study of Rivaroxaban”
Macedonian pharmaceutical bulletin, 69 (Suppl 1) 289 – 290 (2023), Online ISSN 1857 – 8969, DOI: 10.33320/maced.pharm.bull.2023.69.03.140, Short communication, [email protected] S5 PP 01, The influence of directly compressible fillers/diluents on selected compact properties and drug dissolution: a case study of rivaroxaban, Ivana Vasiljević, Paulina Pasik, Erna Turković, Branka Ivković, Arkadiusz Hejduk, Janina Lulek, Jelena Parojčić
See the webinar:
“Rational Selection of Cyclodextrins for the Solubilization of Poorly Soluble Oral Drugs”, 8. November 2023:
Get more information & register here for free: