Targeted colonic release formulations of mesalazine – A clinical pharmaco-scintigraphic proof-of-concept study in healthy subjects and patients with mildly active ulcerative colitis
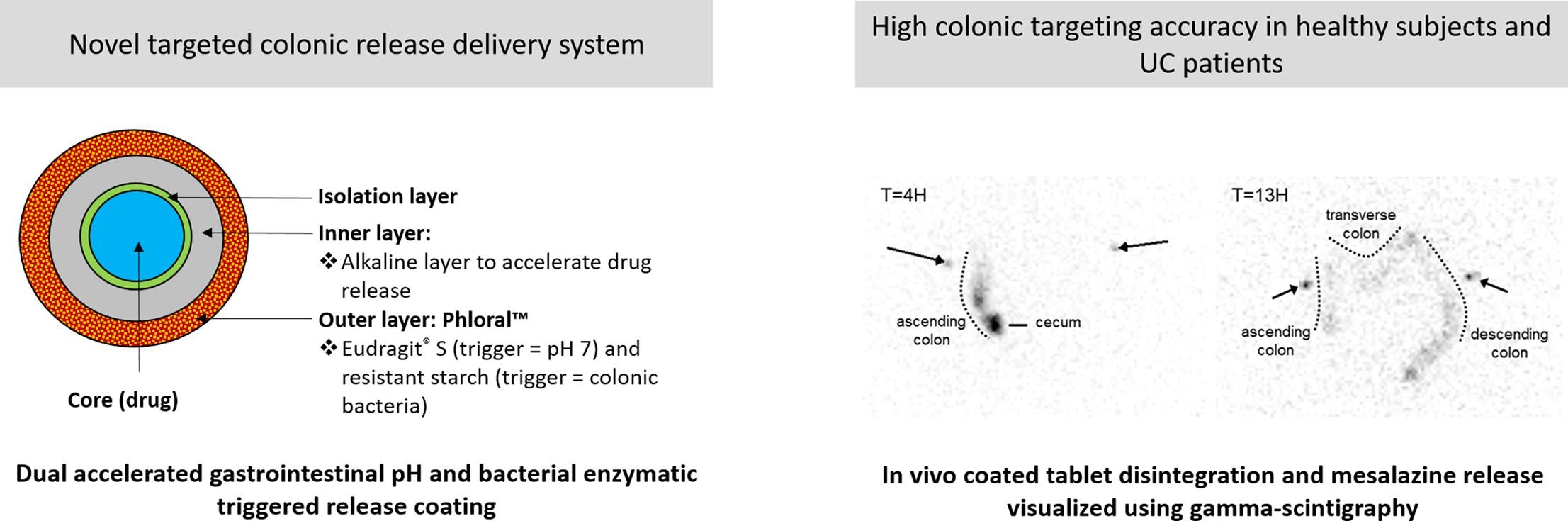
Colonic targeting of orally applied therapeutic drugs remains a challenge. Tablet coatings relying on gastrointestinal pH and colonic bacterial enzymes as triggers in association with an inner alkaline layer are expected to improve targeting efficiency. Mesalazine release from three differently coated tablets labelled with 1 MBq 153Sm was characterised in a single centre, open-label, parallel group study in nineteen healthy subjects and seven patients with mildly active ulcerative colitis. Two semi-organic and one aqueous-based outer coating with different ratios of enteric polymer and resistant starch were tested. All coatings showed comparable release lagtimes in biorelevant dissolution media and were not affected by neutron-activation of the samarium tracer. Mesalazine pharmacokinetics and gamma scintigraphy were used to characterise drug release, anatomical site of tablet disintegration and gastrointestinal transit. Initial tablet disintegration occurred at the ileo-caecal junction or beyond in 92 % of the subjects. Time to initial tablet disintegration was inversely correlated with maximal plasma concentrations and systemic mesalazine exposure. Although high inter-subject variability precluded detection of differences between solvent types and different enteric polymer to polysaccharide ratios, the dual pH and enzymatic triggered release system in combination with an inner alkaline layer promoted mesalazine release at the target site with high accuracy.
Download the full article as PDF here Targeted colonic release formulations of mesalazine – A clinical pharmaco-scintigraphic proof-of-concept study in healthy subjects and patients with mildly active ulcerative colitis
or read it here
Materials
Samarium oxide (99.99 % purity) was obtained from Alfa Aeasar, Kandel, Germany, mesalazine from Pharmazell, Raubling, Germany, hypromellose (Pharmacoat® 603 and Pharmacoat 606) from ShinEtsu, Tokyo, Japan. Microcrystalline cellulose and sodium starch glycolate(Explotab®) were obtained from JRS Pharma, Rosenberg, Germany.
Resistant starch (Amylo N-400) was sourced from Roquette-Fréres, Beinheim, France. Polysorbate 80 (Tween® 80), polyethyleneglycol 6000,magnesium stearate and 1-Butanol were supplied by Merck, Darmstadt, Germany. Ethanol 96 % was purchased from Thermofisher Scientific, Loughborough, UK. Eudragit® S100 and colloidal silica (Aerosil® 200) were obtained from Evonik, Darmstadt, Germany. Triethyl citrate was supplied by Jungbunzlauer, Ladenburg, Germany. Glyceryl monostearate was purchased from Hänseler AG, Herisau Switzerland. Sodium hydroxide was obtained from VWR International, Dietikon, Switzerland, buffer salts used for the preparation of dissolution buffers from Sigma-Aldrich; Buchs, Switzerland.
F. Varum, H. Thorne, R. Bravo, D. Gilgen, C. Hartig, G.P. Nicolas, D. Wild, E. Liakoni, M. Haschke,
Targeted colonic release formulations of mesalazine – A clinical pharmaco-scintigraphic proof-of-concept study in healthy subjects and patients with mildly active ulcerative colitis, International Journal of Pharmaceutics, Volume 625, 2022, 122055, ISSN 0378-5173,
https://doi.org/10.1016/j.ijpharm.2022.122055.
In this study, the Phloral technology was used. See the full brochure on “Phloral“ here:
(click the picture to download the brochure)


