Colouring Orally Dispersible Tablets
Delivering active ingredients via orally dispersible tablets (ODTs) is a recent trend in the pharmaceutical industry. Due to the easy and fast application, patient’s compliance is high for this kind of dosage form. To ensure a fast drug release, ODTs are typically not coated.
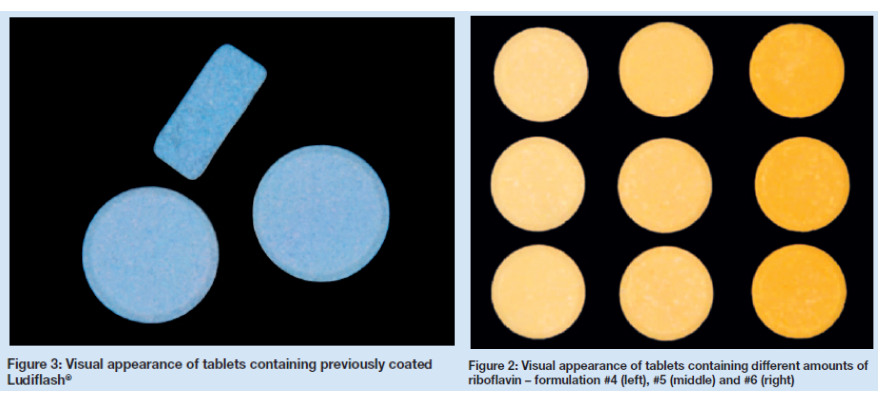
Nevertheless, a product-specific appearance by means of colour might also be requested. This work was to investigate possibilities of how a colorant might be added to an ODT avoiding a film coating process. Two methods were tested: firstly the direct compression (DC) and secondly the application of the colouring agents.
Conclusion: It could be shown that the colouration of the tableting blend is an alternative to the coating of ODTs which bares the risk of prolonging disintegration time. Whether the colouring agent can directly be added to the powder blend or has to be applied previously, is dependent on its nature and the expected hue. However, coating of either Ludifl ash® or the whole powder blend, including the API was found to be an effi cient approach. For achieving a coloured DC material, it could even be considered to add the colouring agent directly to the Kollicoat® SR 30 D dispersion during the production of Ludiflash®.
DOWNLOAD THE POSTER AS A PDF HERE
Materials: Ludiflash® (based on mannitol (90 %), Kollicoat® SR 30 D (5 % solids) and Kollidon® CL-SF (5%)) from BASF SE, Ludwigshafen, Germany, were used. As colouring agents, Indigotine 85 % E132 (Sensient, King’s Lynn, UK) and riboflavin (BASF SE, Ludwigshafen, Germany) were used. As flavours, peppermint (Bell Flavors & Fragrances, Leipzig, Germany) and banana (Symrise AG, Holzminden, Germany) were used. As most suitable lubricant, sodium stearyl fumarate (PRUV®, JRS GmbH & Co. KG, Rosenberg, Germany) was used.


