Study of Compaction Tools and Parameters on Critical Quality Attributes of High Drug Load Minitablets
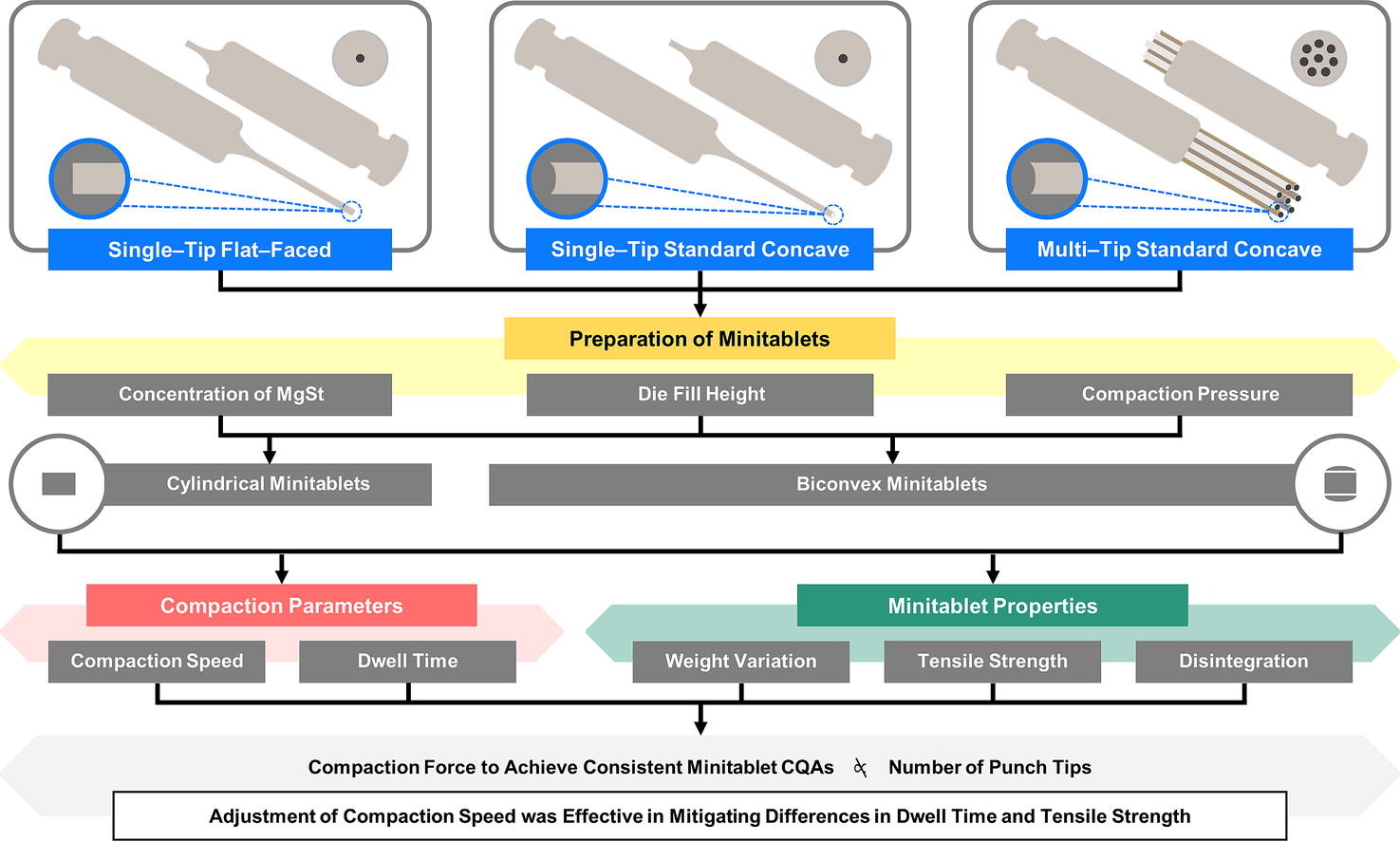
Minitablets are prepared using multiple die openings and multi–tip punches for greater productivity. With multiple tips on the punch barrel, the overall compaction force to be applied is commonly estimated by multiplying the desired compaction force per tip by the number of punch tips. Few researchers have however examined this proportionality and the effects of the number of punch tips and punch face geometry on the critical quality attributes (CQAs) of high drug load minitablets. In this study, the minitablets prepared by multi–tip tools exhibited greater weight variation than those prepared by single–tip tools. Their compaction was accompanied by a longer dwell time that led to a higher minitablet tensile strength and consequently a longer disintegration time.
The compaction forces required to achieve a consistent set of minitablet CQAs were not directly proportional to the number of punch tips used. In comparison, the effect of punch face geometry was negligible. Increasing concentration of magnesium stearate (as lubricant) from 0.75 to 1.25 %, w/w reduced weight variation, especially of minitablets prepared by the multi-tip tools. It also increased the disintegration time but had no significant effect on the tensile strength of the minitablets regardless of type of tools used. The adjustment of compaction speed was an effective compensatory method to mitigate the differences in dwell time and tensile strength between minitablets prepared by single–tip and multi–tip standard concave tools. A larger reduction in compaction speed of the single-tip tools was required at higher compaction pressures.
Read the full article here
Materials
Paracetamol (PCM; Granules India Limited, India) was used as the model drug in this study. Polyvinylpyrrolidone (PVP; Kollidon K30, BASF, Germany), sodium starch glycolate (SSG; Primojel, DFE Pharma, Germany) and microcrystalline cellulose (MCC; Ceolus PH101, Asahi Kasei, Japan) were excipients used at 5 %, w/w, 1 %, w/w and 9 %, w/w respectively for the preparation of diminutive high shear granules containing a high drug load of 85 %, w/w PCM.
Shang Jun Loo, Cheng Yee Lim, Paul Wan Sia Heng, Lai Wah Chan, Study of Compaction Tools and Parameters on Critical Quality Attributes of High Drug Load Minitablets, International Journal of Pharmaceutics, 2024, 123806, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.123806.
Read also the article Comparison of Ceolus™ Grades in Continuous Manufacturing (Wet Granulation) – Continuous Manufacturing System “Granuformer® Gf-2050” here: