Towards the Continuous Manufacturing of Liquisolid Tablets Containing Simethicone and Loperamide Hydrochloride with the Use of a Twin-Screw Granulator
Abstract
Continuous manufacturing is becoming the new technological standard in the pharmaceutical industry. In this work, a twin-screw processor was employed for the continuous production of liquisolid tablets containing either simethicone or a combination of simethicone with loperamide hydrochloride. Both active ingredients present major technological challenges, as simethicone is a liquid, oily substance, and loperamide hydrochloride was used in a very small amount (0.27% w/w). Despite these difficulties, the use of porous tribasic calcium phosphate as a carrier and the adjustment of the settings of the twin-screw processor enabled the optimization of the characteristics of the liquid-loaded powders and made it possible to efficiently produce liquisolid tablets with advantages in physical and functional properties. The application of chemical imaging by means of Raman spectroscopy allowed for the visualization of differences in the distribution of individual components of the formulations. This proved to be a very effective tool for identifying the optimum technology to produce a drug product.
Introduction
The continuous manufacturing (CM) of pharmaceutical products is becoming a new technological standard in the pharmaceutical industry. This technology is very promising, so such regulatory bodies as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) support its development and implementation as a way to modernize pharmaceutical manufacturing and improve product quality [1,2]. Today, most pharmaceutical products are produced by a method known as batch manufacturing in which different operations, such as weighing, mixing, tableting, or encapsulation, are performed in separate rooms and at different times. This is a long, multi-step process that requires large-scale equipment. CM is faster than batch processing and easier to scale. It eliminates the need to stop and move intermediates between production steps, which can potentially improve the quality of the final pharmaceutical product. Therefore, leaders in the pharmaceutical industry are interested in this technology, which compared to the traditional manufacturing process, speeds up the production cycle, improves overall efficiency, and significantly reduces costs [3,4].
Of the various granulation techniques, twin-screw granulation (TSG) appears to be the most flexible and efficient for CM processes [5,6,7]. It enables the continuous feeding of raw materials, their processing, and uninterrupted transfer to subsequent unit processes. Product quality attributes can be adjusted and optimized just by changing the TSG settings and/or process parameters. The amount of manufactured product can be easily regulated by shortening or lengthening the processing time [8,9,10]. Compared to other granulation equipment, TSG offers the possibility of true continuous production. Very often, processes with the use of a high-shear granulator (HSG) or fluid-bed granulator (FBG) are run in a so-called mini-batches mode. Strictly speaking, these processes are, therefore, rather semi-continuous but are still covered by the relevant ICH or FDA guidelines [1,2].
In this study, a twin-screw granulator was utilized as a continuous processor in the production of liquisolid tablets containing either simethicone or a combination of simethicone and loperamide hydrochloride.
Simethicone is a mixture of polydimethylsiloxane and silicon dioxide and is commonly used as a defoaming agent to relieve abdominal discomfort caused by excessive intestinal gas (e.g., bloating, cramping, flatulence). Simethicone occurs as a hydrophobic, semiopaque, viscous fluid [11]. Loperamide is an effective antidiarrheal drug that is widely administered for the symptomatic treatment of various types of diarrhea [12,13]. The combination of loperamide and simethicone is used to treat acute diarrhea combined with abdominal distension and pain. Loperamide and simethicone show synergistic effects, and their combination is more effective in relieving acute diarrhea than when taken alone [14,15,16]. Such combinations containing 2 mg of loperamide hydrochloride and 125 mg of simethicone are already available on the market [17,18].
The liquisolid technique consists of absorbing a drug in a liquid state by a porous carrier and converting it into a dry, free-flowing, non-adherent powder that is suitable for tableting [19,20]. So, the liquid drug must first be incorporated into the porous structure of a carrier by both absorption and adsorption (collectively referred to as sorption). Thus, the properties of a carrier material, including an elevated specific surface area (SSA) and high loading capacity, play a major role in the preparation of a dry liquid-loaded powder. In pharmaceutical technology, excipients such as magnesium aluminometasilicate, dibasic calcium phosphate, mesoporous calcium silicate, mesoporous magnesium carbonate, or mesoporous silica are often used as carrier material. For some water-insoluble carriers, the incomplete release of certain drugs and even the possibility of drug re-adsorption on the carrier surface have been observed, which may limit their use in solid oral drug formulations [21,22,23]. It has been reported that this technique requires the additional use of very fine and highly adsorptive materials such as silicon dioxide, calcium silicate, or magnesium aluminometasilicates [24,25,26,27,28].
The liquisolid technique is quite versatile, and many examples of its use for the preparation of oral solid dosage forms can be found in the scientific literature. Limpongsa et al. demonstrated its successful application for the preparation of directly compressible orally disintegrating tablets containing hydrophobic cannabinoids [29]. In their study, Kostelanská et al. demonstrated the increased solubility and dissolution rate of liquisolid tablets containing plant extracts [30], and Devi et al. evaluated the feasibility of liquisolid formulations to improve the dissolution rate of ketoprofen and, thus, its bioavailability [31]. Other papers have also reported the applicability of this technology to improve the solubility of poorly soluble drug substances such as triclabendazole or mirtazapine [32,33].
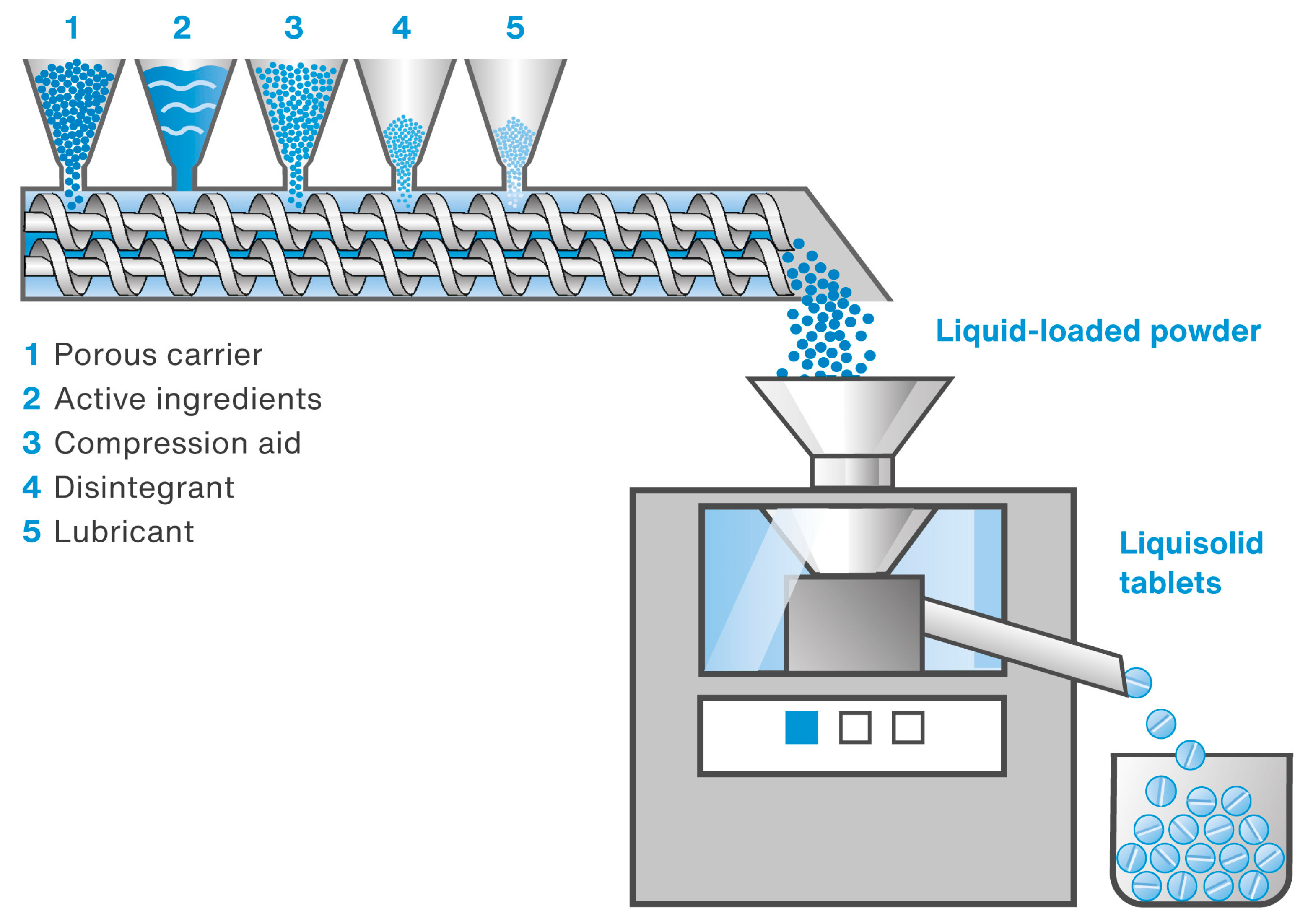
The main objective of the present study was to investigate the possibility of changing the production of liquisolid tablets containing simethicone (as well as a combination of simethicone and loperamide hydrochloride) from a batch process to a continuous one. The continuous process involved preparing liquid-loaded powders using TSG and directly compressing them into tablets on a rotary tablet press. The device used (Thermo ScientificTM Pharma 16 Twin-Screw Extruder, Thermo Fisher Scientific, Karlsruhe, Germany) enabled the simultaneous and continuous feeding of all formulation components (both the liquid and powders). A schematic diagram of the equipment set used in the present study is shown in Figure 1.
Download the full article as PDF here: Towards the Continuous Manufacturing of Liquisolid Tablets Containing Simethicone and Loperamide Hydrochloride with the Use of a Twin-Screw Granulator
or read it here
Materials
Simethicone Q7-2243 LVA from Dow Corning GmbH (Wiesbaden, Germany). Loperamide hydrochloride from Vasudha Pharma Chem Limited (Hyderabad, India). Tribasic calcium phosphate, TCP (TRI-CAFOS® 500), and anhydrous dibasic calcium phosphate, DCPA (DI-CAFOS® A150) from Chemische Fabrik Budenheim KG (Budenheim, Germany). Croscarmellose sodium (Ac-Di-Sol® SD-711 NF) from FMC BioPolymer (Philadelphia, PA, USA) and magnesium stearate (Ligamed® MF-2-V) from Peter Greven Fett-Chemi (Venlo, The Netherlands).
Zakowiecki, D.; Richter, M.; Yuece, C.; Voelp, A.; Ries, M.; Papaioannou, M.; Edinger, P.; Hess, T.; Mojsiewicz-Pieńkowska, K.; Cal, K. Towards the Continuous Manufacturing of Liquisolid Tablets Containing Simethicone and Loperamide Hydrochloride with the Use of a Twin-Screw Granulator. Pharmaceutics 2023, 15, 1265. https://doi.org/10.3390/pharmaceutics15041265
Read more on Magnesium Stearate as a pharmaceutical excipient here:


