Preparation of core-shell controlled release tablets using direct powder extrusion 3D printing techniques
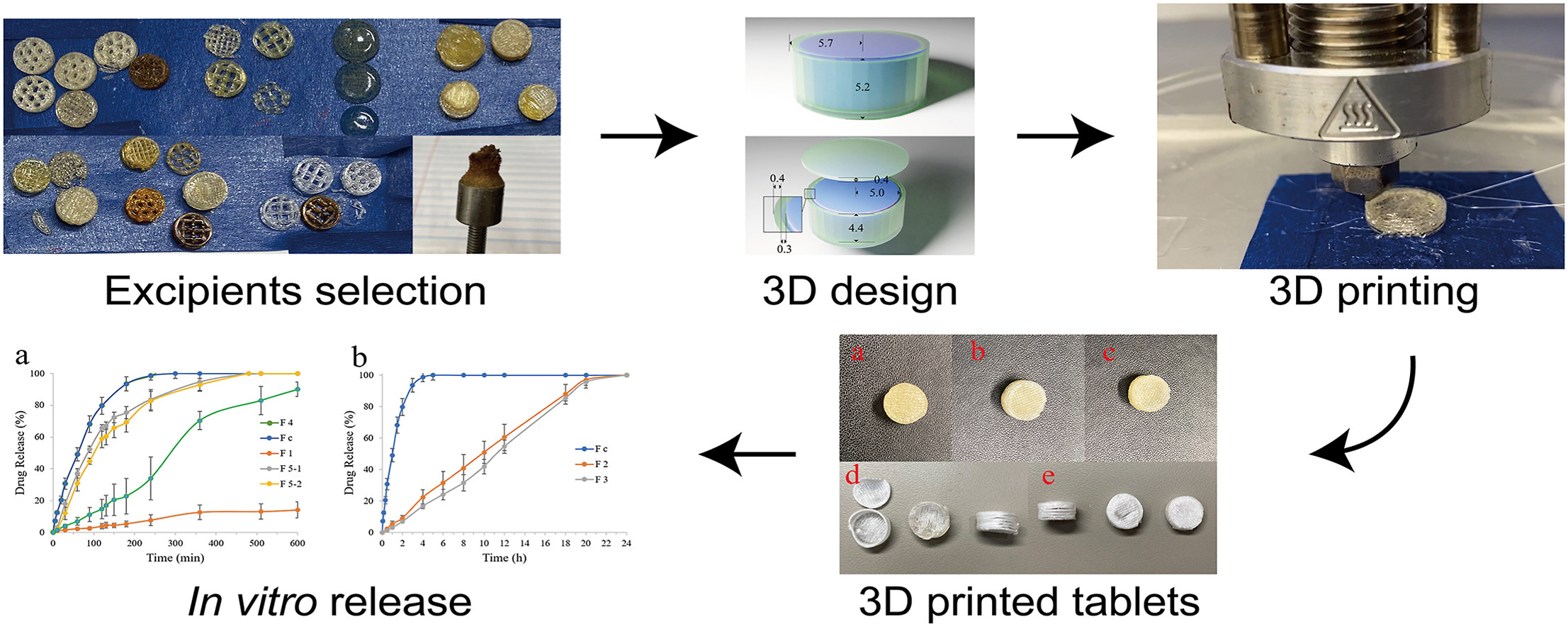
Three-dimensional printing (3DP) is a widely used technique to manufacture drug delivery systems due to its precision, efficiency, and tailored prescription capability. Although fused deposition modeling (FDM) is the most commonly used technique in the pharmaceutical sciences, there are several limitations. Direct powder extrusion (DPE) 3D printing is a novel technique that was improved from traditional FDM. Material powder blends are fed into the cartridges of the 3D printer directly, without filament manufacturing, avoiding investigation and modification of filament property improvement. The present work was to fabricate core-shell geometry controlled-release tablets via direct powder extrusion 3D printing. A 3D tablet structure with a core surrounded by a shell was successfully designed. Acetaminophen (APAP) was used as the model drug, and Kollidon® VA64 was chosen as a filler, which composed the core. Eudragit® E PO, HPMC AS LG, and Eudragit® RS PO polymers were employed to print the shell. Two cartridges were utilized to load ingredient mixtures and print simultaneously. Modifications were made to printing parameters, such as printing temperature, pressure, and speed. Differential scanning calorimetry thermograms and Powder X-ray diffraction indicated the transformation of APAP from its crystalline to the amorphous state. The 3D-printed tablet without a shell showed complete drug release in 5 h. In contrast, the tablets with pure Eudragit® E PO and HPMC AS LG polymer combination demonstrated extended rather than delayed release. The dissolution profile of the tablets coated with Eudragit® RS PO mixed with PEG 4500 showed zero-order controlled release, which exhibited complete drug release in 20 h.
Materials
Acetaminophen (APAP) was purchased from SpecGx LLC (Raleigh, NC, USA). AquaSolve™ hypromellose acetate succinate (HPMC-AS) LG was donated by Ashland Inc. (Covington, KY, USA). Kollidon® VA 64 was donated by BASF (Ludwigshafen, Germany). Eudragit® E PO, and Eudragit® RS PO were donated by Evonik Industries (Essen, Germany). Polyethylene glycol (PEG) 4000 was purchased from Avantor Performance Materials, Inc. (Center Valley, PA, USA). All other reagents were of analytical grade.
Read more
Honghe Wang, Sateesh Kumar Vemula, Suresh Bandari, Michael A. Repka, Preparation of core-shell controlled release tablets using direct powder extrusion 3D printing techniques, Journal of Drug Delivery Science and Technology, Volume 88, 2023, 104896, ISSN 1773-2247,
https://doi.org/10.1016/j.jddst.2023.104896.

