Aptamer-modified chitosan-capped Mesoporous silica nanoparticles for co-delivery of cytarabine and daunorubicin in Leukemia
Abstract
In this study, surface modified mesoporous silica nanoparticles (MSNs) were prepared for the targeted delivery of the anticancer agents, daunorubicin (DNR) and cytarabine (CTR), against K562 leukemia cancer cell lines. The MSNs were surface-modified with pH-sensitive chitosan (CS) to prevent the burst release of anticancer agents at the physiological pH of 7.4 and to enable a higher drug release at lower pH and higher concentration of glutathione. Finally, the MSNs were surface modified with KK1B10 aptamer (Apt) to enhance their uptake by K562 cells through ligand-receptor interactions. The MSNs were characterized using different methods and both in vitro and in vivo experiments were utilized to demonstrate their suitability as targeted anticancer agents. The resultant MSNs exhibited an average particle size of 295 nm, a surface area of 39.06 m2/g, and a cumulative pore volume of 0.09 cm3/g. Surface modification of MSNs with chitosan (CS) resulted in a more regulated and acceptable continuous release rate of DNR.
The drug release rate was significantly higher at pH 5 media enriched with glutathione, compared to pH 7.4. Furthermore, MSNs coated with CS and conjugated with aptamer (MSN-DNR+CTR@CS-Apt) exhibited a lower IC50 value of 2.34 µg/ml, compared to MSNs without aptamer conjugation, which displayed an IC50 value of 12.27 µg/ml. The results of the cell cycle analysis indicated that the administration of MSN-DNR+CTR@CS-Apt led to a significant increase in the population of apoptotic cells in the sub-G1 phase. Additionally, the treatment arrested the remaining cells in various other phases of the cell cycle. Furthermore, the interactions between Apt-receptors were found to enhance the uptake of MSNs by cancer cells. The results of in vivo studies demonstrated that the administration of MSN-DNR+CTR@CS-Apt led to a significant reduction in the expression levels of CD71 and CD235a markers, as compared to MSN-DNR+CTR@CS (p<0.001). In conclusion, the surface modified MSNs prepared in this study showed lower IC50 against cancer cell lines and higher anticancer activity in animal models.
Introduction
Leukemia, a hematologic malignancy that affects the lymphatic system, bone marrow, and blood cells, is a particularly common cancer in adults (Aaryasomayajula et al., 2014). Acute lymphocytic leukemia (ALL), Chronic myeloid leukemia (CML), chronic lymphocytic leukemia (CLL), and acute myeloid leukemia (AML) are all kinds of leukemia. AML is a hematologic malignancy that is the most prevalent leukemia among adults and more dangerous in older individuals, with a higher incidence rate and over 90% fatality rate in those over the age of 65, as well as a variety of clinical presentations and classifications (Acharya and Kala, 2019, Ahsan et al., 2018). Chemotherapy is expensive, harmful, and causes cancer resistance (Alibolandi et al., 2015). Despite advances in pathobiology and innovative therapeutic targets, traditional AML induction treatments have not changed significantly in decades (Aliyu et al., 2020). Additionally, when chemoresistance adverse effects such as myelosuppression, cardiotoxicity, and infections are present, conventional treatments always end in treatment failure or relapse, resulting a poor overall prognosis. Hence, new therapeutic approaches should be developed (Aliyu et al., 2020, Aliyu et al., 2019).
Nano-based drug delivery systems have the potential to deliver pharmaceuticals more efficiently and with fewer side effects (Ambrogio et al., 2011, Amin and Boateng, 2020). Furthermore, by conjugating medicines with diverse ligands, medications could target AML cell-surface receptors, thereby improving therapeutic efficacy or overall survival (OS) rates significantly (Bala et al., 2000, Bhattarai et al., 2010).
Mesoporous silica nanoparticles (MSN) with average particle sizes in the range of 50-300 nm and narrow pore size distributions of 3-6 nm are gaining popularity among scientists for use as drug delivery systems (Blair, 2018). MSN have appealing properties such as the capacity to adjust particle size, the possibility of functionalizing the silica surface, degradability under physiological conditions, biocompatibility, and pore volumes and large surface areas, which allow for the entrapment of huge numbers of cargo molecules (Carulli et al., 2018, Castillo et al., 2020). It is also conceivable to give MSN with smart medication delivery capability by blocking the pore entrances with appropriate gatekeepers that prohibit the cargo from being released before it reaches the target. When exposed to internal or external stimuli, the nanogates open, allowing the entrapped cargo to escape (Castillo et al., 2020, Chai et al., 2011). Several gatekeepers are built based on the stimuli that will be employed as a release trigger. The stimuli employed can be either internal (redox potential, pH, biomolecules, temperature) or external (magnetic field, light), or a mix of both (multiresponsive or dual-stimuli release systems) (Chambers and Mitragotri, 2004, Chang et al., 2007). MSN are generally much more resistant to environmental factors inside the body, however, when sensitive bonds that can be decomposed under special conditions are introduced into its structure, MSN are decomposed under special conditions, and the loaded drugs are released. For example, when the monomer Bis [3-triethoxysilylpropyl] disulfide is used in the synthesis of MSNs, they would be decomposed in the presence of reducing agents due to the presence of the disulfide bond (Chen et al., 2019).
The utilization of mesoporous silica nanoparticles (MSNs) in the development of drug delivery agents has gained popularity due to their facile synthesis process, high surface area and pore volume, facile single-step functionalization, customizable particle size and morphology, favorable physicochemical stability, and biocompatibility (Colilla et al., 2013). The size of MSNs plays a crucial role in enhancing their stability and blood circulation time. To ensure a prolonged circulation half-life, the preferred size range for MSNs is between 50-300 nm (Croisier and Jérôme, 2013). The lower limit is set to prevent rapid renal clearance, while the upper limit is established to avoid embolisms resulting from aggregation in capillaries and alveoli. Surface modification of nanocarriers has been shown to extend their blood circulation time by evading macrophages and achieving target recognition and localization (Croissant et al., 2014). Additionally, the attachment of nanoparticles to the surface of red blood cells has been proposed as a means of prolonging circulation time and achieving sustained release of therapeutic agents (Deng et al., 2011, Estey, 2018).
The difference in redox potential between healthy and cancer cells has provided a new strategy for drug delivery. This approach is based on the presence of different reducing species inside the cell such as glutathione (GSH), nicotinamide adenine dinucleotide (NADH+), and dihydrolipoic acid (DHLA) (Fajardo et al., 2013) .GSH is the most abundant thiol species in the cytoplasm and the main reducing agent in biochemical processes. The concentration of GSH inside the cell is 100-1000 times higher than outside the cell (Farge et al., 2017).
Chitosan (CS), a natural cationic polysaccharide comprised of β-(1–4)-linked glucosamine units and some N-acetyl-D-glucosamine units, is produced via deacetylation of chitin (Forman et al., 2009) .It has been used in a variety of biomedical and drug delivery systems due to its biodegradable, nontoxic, and antibacterial qualities (Gan et al., 2011, Gan et al., 2015). In order to have the physicochemical properties of swelling, retention of water and adsorption, the secondary structure of chitosan is formed by a hydroxyl group, amino group and N.acetyl group on the molecular chain involved in the formation of intramolecular and intermolecular hydrogen bonds of chitosan (Gerson et al., 1986). The C2-NH2, C3-OH and C6-OH functional groups of chitosan can form stable covalent bonds via several reactions such as etherification, acetylation, esterification ,acylation, phosphorylation, Schiff’s bases, cross-linking modification and reductive amination reactions (Guo et al., 2018, Hall et al., 2007).
Because the amino groups in CS are protonated at a certain pH range, CS can respond to external pH stimulation. Because CS has a pKa of 6.3, its pH response is slightly acidic. The microenvironment inside cancers cells has an acidic pH of 5-6.8, with tumors possessing a lesser extracellular pH than healthy tissue (Hanafi-Bojd et al., 2018, He et al., 2014). The degree of deacetylation and molecular weight is very important in determining the Chitosan properties. The positive charge of Chitosan may also bind to negative charge in red blood cells RBC, so that chitosan is used as a haemostatic agent (Helalat et al., 2023). As a result, CS is a promising biopolymer for providing MSN with a pH responsive polyelectrolyte layer to limit the release of loaded anticancer medications to tumor tissues’ acidic local environment.
For more than three decades, combination chemotherapy with cytarabine (CTR) and daunorubicin (DNR) has been one of the most effective induction therapies for acute myelogenous leukemia (AML) (Heydari et al., 2023). Both medications have also been demonstrated to be effective against ALL (Huang et al., 2019). DNR has cytotoxic activity and antimitotic via a variety of mechanisms, such as suppression of topoisomerase II activity, complex formation with DNA, gene expression control, inhibition of DNA polymerase activity, and creation of DNA-damaging free radicals. CTR has an effect on cells exclusively during the S phase of cell division and works primarily by inhibiting DNA polymerase (Islam et al., 2015). Therefore, targeting the CTR and DNR combination to cancer cells will be a major achievement in treatment of ALL.
Various targeting moieties have been applied for cancer cells. One of the promising strategy is the use of aptamer. Aptamers (Apt) are single-stranded DNA or RNA oligonucleotides that have the ability to bind to their target with high affinity and specificity (Jain et al., 2012). Apt are widely used as new biomedical tools for analytical applications, drug delivery imaging, etc (Karimifard et al., 2022). They are able to connect to nanoparticle systems and act as a ligand in diagnosis and drug delivery to the cancer tumor site (Kennedy et al., 2020). Apt have many advantages, including easy chemical synthesis, non-immunity, small size to enter biological components, and chemical binding with functional groups (Khodabandehloo et al., 2016). Apt are isolated using a repetitive protocol or systematic evolution of ligands by exponential enrichment (SELEX) for different purposes (Kim et al., 2011). KK1B10 is a DNA Apt, more specific for K562 cell line, identify targets related to monocytic targets (Kollia et al., 2001).
Aptamers possess unique characteristics, including small size, high stability, structural flexibility, ease of synthesis, and low or no immunogenicity, which render them an excellent targeting agent (Kurrikoff et al., 2019). To enhance their circulation half-life (t1/2), chemical modification of the aptamer’s nucleic acid chain or conjugation with coupling agents can be employed to achieve prolonged action (Lakhin et al., (aнглoязычнaя вepcия). 2013). Aptamer modification aims to increase their stability against nuclease degradation or prolong their half-life against kidney filtration, and can be performed during or after selection. The former approach seeks to stabilize aptamers against nucleases through 3′-inverted thymidine, which significantly increases their stability and resistance to exonuclease in circulation. Additionally, attaching aptamers to nanoparticles can favorably increase their stability. Conjugating aptamers with nanomaterials can extend circulation time and reduce degradation (Lamarra et al., 2018).
N-ethyl-n-nitrosourea (ENU), is considered a powerful carcinogen that can induce leukemia by transferring an alkyl group to a nucleobase, therefore serious causing DNA changes, In many cases Prolonged myelosuppression and subsequent leukemogenesis (Li et al., 2017, Liu et al., 2012).
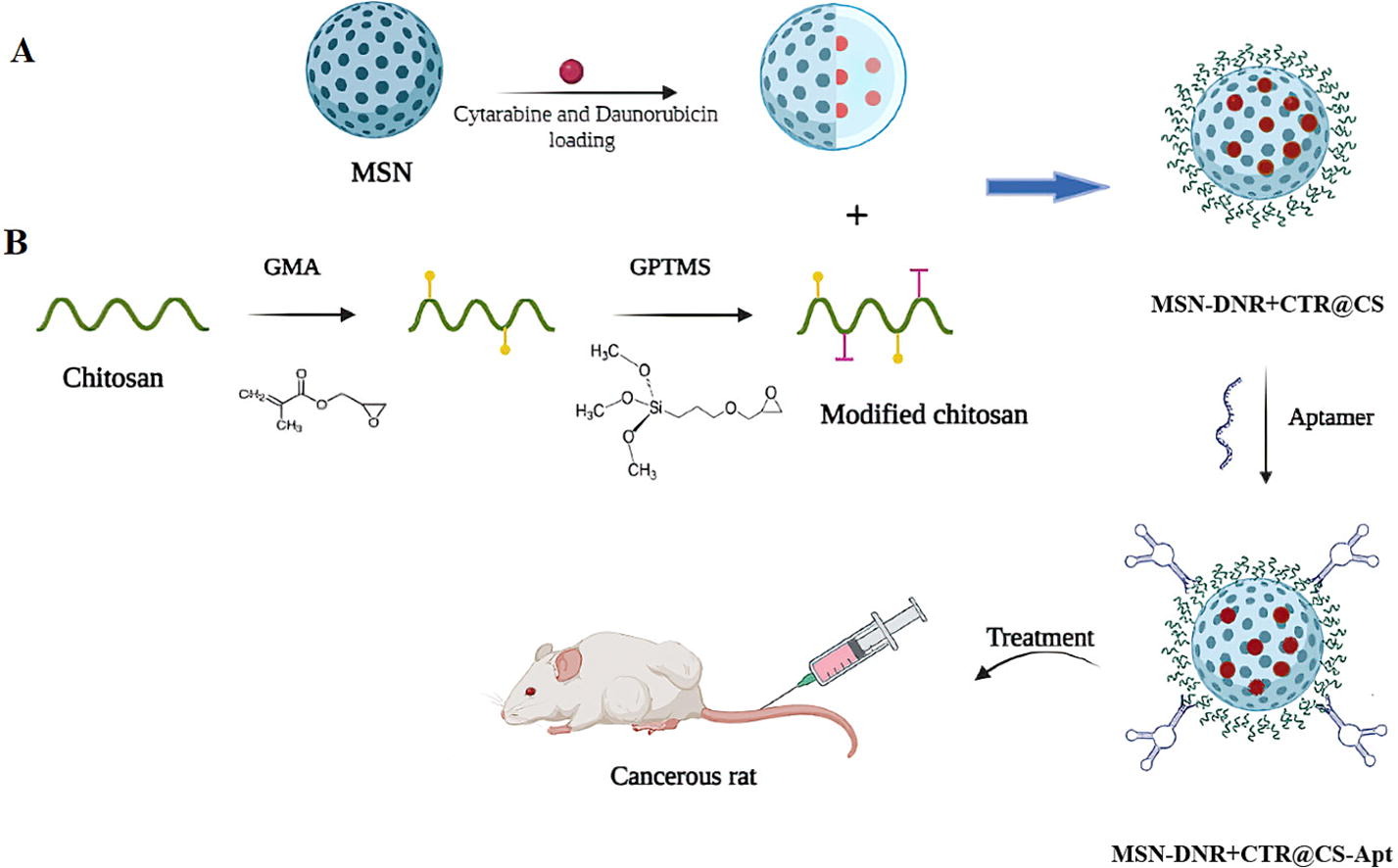
In the current study, monodispersed MSN were fabricated and loaded with DNR and CTR (MSN-DNR+CTR) then coated them with CS (MSN-DNR+CTR@CS).pH-sensitive MSN-DNR+CTR@CS were conjugated with Apt (MSN-DNR+CTR@CS-Apt) to produce the desired nanoparticles for targeted drug delivery to leukemia cells. We introduced the disulfide bridge within MSN to facilitate intracellular degradation of GSH. In addition, CS coating is used to prevent drug release and allow particle decomposition under acidic conditions. Also, conjugating aptamer to chitosan surface can be effective in nanoparticle targeting.
Read more here
Seyed Reza Heydari, Mohammad Hossein Ghahremani, Fatemeh Atyabi, Reza Bafkary, Mahmoud Reza Jaafari, Rassoul Dinarvand, Aptamer-modified chitosan-capped Mesoporous silica nanoparticles for co-delivery of cytarabine and daunorubicin in Leukemia, International Journal of Pharmaceutics, 2023, 123495, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123495
See the webinar:
“Rational Selection of Cyclodextrins for the Solubilization of Poorly Soluble Oral Drugs”, 8. November 2023:
Get more information & register here for free:


