A validation of discrete-element model simulations for predicting tablet coating variability
Abstract
Achieving an even coating distribution on tablets during the coating process can be challenging, not to mention the challenges of accurately measuring and quantifying inter-tablet coating variability. Computer simulations using the Discrete Element Method (DEM) provide a viable pathway towards model-predictive design of coating processes. The purpose of this study was to assess their predictivity accounting for both experimental and simulation input uncertainties. To this end, a comprehensive set of coating experiments covering various process scales, process conditions and tablet shapes were conducted. A water-soluble formulation was developed to enable rapid spectroscopic UV/VIS analysis of coating amounts on a large number of tablets. DEM predictions are found to lie within the experimentally inferred confidence intervals in all cases. A mean absolute comparison error of 0.54 % was found between model predictions of coating variability and respective sample point estimates. Among all simulation inputs the parameterization of spray area sizes is considered the most significant source for prediction errors. However, this error was found significantly smaller in magnitude compared to experimental uncertainties at larger process scales underlining the value of DEM in the design of industrial coating processes.
Introduction
The coating is an important part of solid dosage forms, particularly tablets. Coatings may serve various purposes, such as active ingredient protection, release profile modification, or cosmetic appearance improvement to safeguard patient compliance (Seo et al., 2020). The thickness of a coating is a crucial parameter that affects its cosmetic or therapeutic function and thus should be in the desired range for the entire population of tablets in a batch. It follows that the uniformity of film coverage, henceforth quantified by inter-tablet coating variability, is a critical quality attribute that should be well understood and controlled. This necessitates the ability to directly measure, statistically infer or predict coating variabilities.
The amount of coating on a tablet, either specified by coating thinkness or mass, can be quantified using several analytical techniques. However, to determine the coating variability of an entire batch with narrow confidence interval a large amount of tablet samples must be measured. This renders many analytical techniques, such as Terahertz pulsed imaging (Brock et al., 2012), X-ray micro-computed tomography, and confocal microscopy, while useful in quantifying intra-tablet coating variability on single tablets (Radtke et al., 2019, Brock et al., 2013, Brock et al., 2014), unsuitable for the purpose of determining batch coating variabilities. Spectroscopic in-line methods such as near-infrared spectroscopy, on the other hand, require multivariate calibration using reference samples of well-defined coating amounts, which is often infeasible. Naïve approaches, such as the direct measurement of height, diameter or weight gains using calipers, screw gauges or weighing scales, respectively, must be assumed susceptible to systematic error sources such as tablet core friability, moisture absorption or operator influences. Recent advances in optical coherence tomography (OCT) for the on-line imaging of coating films (Markl et al., 2015, Lin et al., 2015, Wahl et al., 2019) are promising. However, the applicability of OCT to a general class of coating formulations that may include suspended solid particles, such as color pigments or talcum, remains to be demonstrated.
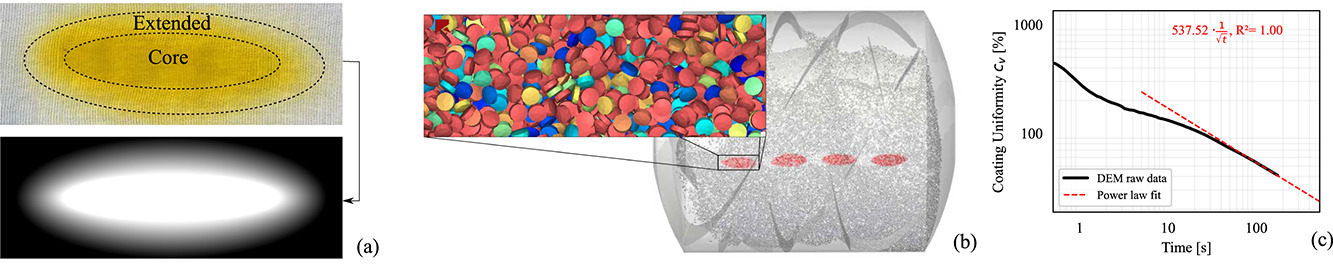
Difficulties associated with measuring coating variabilities motivate model-based predictions thereof. Established models for predicting coating variability can be classified into stochastic and mechanistic approaches. Stochastic modeling approaches based on renewal theory (Mann, 1983, Freireich and Li, 2013), Bernoulli processes (Kalbag and Wassgren, 2009, Joglekar et al., 2007) and Monte-Carlo simulations (Pandey et al., 2006) have provided valuable insights into the interplay between tablet mixing, spray area and the kinetics by which coating variability evolves over coating time. As stated by Freireich and Li (2013), among these insights, the most robust result is that coating variability decreases inversely proportional to the square root of coating time. This particularly applies if the residence time of tablets in the spray area and the recirculation time between passes through the spray area are considered normally and geometrically distributed random variables, respectively. However, a quantitative prediction of using stochastic models requires suitable parameterization of the random variables. This is often unfeasible given the sensitivity of these variables to equipment, process and dosage form. For this reason, significant research attention has been devoted to mechanistic models, specifically the Discrete Element Method (DEM), which can be parametrized based on material properties of the dosage form only.
For recent reviews of DEM applications in the pharmaceutical industry, the interested reader is referred to Yeom et al. (2019) and Russell et al. (2022). A number of studies on DEM simulation of tablet coating processes which also cover experimental validation have been reported in literature. Kalbag et al. (2008) and Ketterhagen (2011) simulated the motion of tablets in coating pans and validated modeling results using a machine vision system to track the trajectories of tracer tablets. Toschkoff et al. (2015) investigated the sensitivity of predicted coating variability to process parameters with particular emphasis on the configuration of spray nozzles. These modeling results match well with data gathered in a previous factorial design of experiments (Just et al., 2013) and the corresponding response surface model for coating variability. While the above studies were limited to laboratory scale, Boehling et al. (2016) reported on the experimental validation of coating variability predictions for production scale processes. Besides predicting coating variability, the vast amount of data generated using DEM, such as contact forces, tablet velocities, and orientations can be used to gain further process insights. Recently, Ketterhagen et al. (2021) used predicted impact energies acting on tablets together with a probabilistic damage model to quantify and mitigate the risk of mechanical tablet damage, e.g. chipping and breakage, in production scale processes. Freireich et al. (2015) and Pei and Elliott (2017) numerically investigated the effect of tablet shape and orientation distributions in spray areas on intra-tablet coating variabilities.
The above references suggests that general consensus has been established in terms of acceptable DEM modeling assumptions, such as the selection and parameterization of contact models which govern the simulated mechanical interaction between tablets. However, to further motivate the use of DEM in the design of production processes and to enable its use in regulatory review processes, more effort should be devoted to model validation. This particularly applies to coating variability where the assessment of model predictivity should encompass experimental uncertainties in light of the aforementioned analytical difficulties. The purpose of this paper is to contribute towards this goal. To this end, a comprehensive set of tablet coating experiments was performed, covering various scales, coating technologies, tablet shapes and process conditions. A water-soluble tablet formulation was developed specifically for the purpose of rapid and accurate spectroscopic analysis of coating amounts. Batch coating uniformities were inferred accounting for experimental uncertainties to enable the assessment of model predictivity.
Read more
Rakulan Sivanesapillai, Anja Ehrig, Leon White Nogueira, Branko Vukosavljevic, Blaž Grilc, Ilija German Ilić, Rahul Bharadwaj, Rok Sibanc, A validation of discrete-element model simulations for predicting tablet coating variability,
International Journal of Pharmaceutics, 2023, 123109, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123109.
Visit our new Webinar:
Solving capping challenges using mannitol as an excipient model
Get more information & register here:


