Dispensing Oral Temozolomide in Children: Precision and Stability of a Novel and Ready to Use Liquid Formulation in Comparison with Capsule Derived Mixtures
Abstract
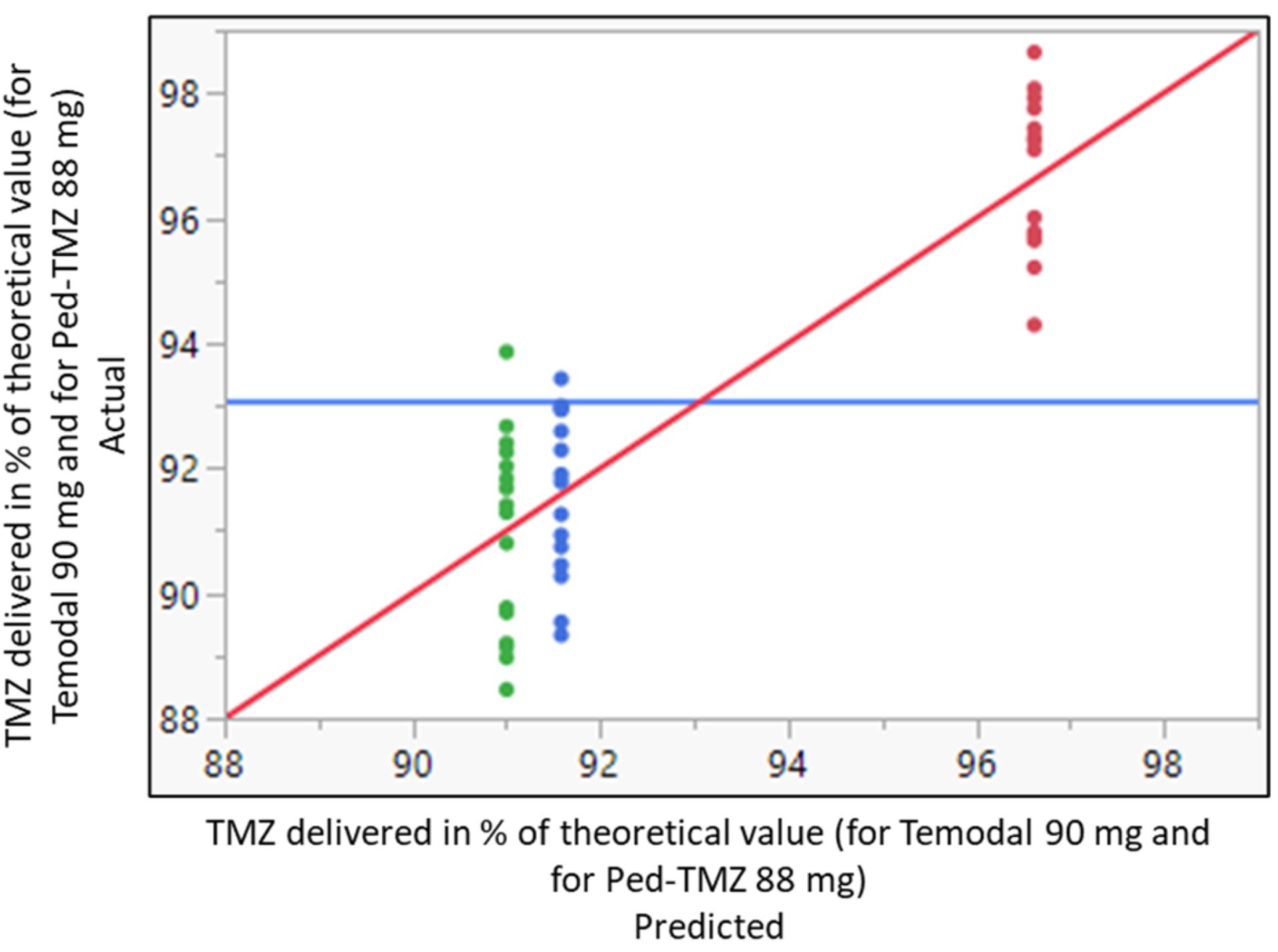
Temozolomide (TMZ) is part of the therapeutic armamentarium used in managing pediatric cancers; however, available oral forms (capsules) are not adapted for use in children. Our aim was to assess the dose accuracy and stability of TMZ using capsule contents mixed with food compared with a novel, ready-to-use liquid formulation specifically developed for children (Ped-TMZ, brand name KIZFIZO). Dose accuracy and TMZ stability testing were performed with TMZ capsule contents (90 mg) mixed with food vehicles (apple juice, apple sauce, cream, milk, and mashed potatoes) and compared to an equivalent dose of Ped-TMZ. Acceptance criteria were predefined for TMZ (95.0–105.0%) and its degradation product amino-imidazole-carboxamide (AIC; <1%) content. The delivered dose was significantly higher using Ped-TMZ (96.6 ± 1.2%) and within the predefined criteria for TMZ content, whereas it was systematically under the lower specifications of 95% using capsule-derived preparations with apple juice (91.0 ± 1.5%) and apple sauce (91.6 ± 1.4%), respectively (p < 0.0001). In chemical stability tests, the four food vehicles (apple sauce, cream, milk, mashed potatoes) had a significant effect on TMZ stability (p = 0.0042), and the AIC significantly increased with time in three of the four vehicles (p < 0.0001). Only 1/72 of preparations from capsules met the predefined acceptance criteria, whereas Ped-TMZ showed no TMZ loss, and the AIC remained within specifications. In conclusion, mixing TMZ capsule content with food may result in significant underexposure, possibly even greater in routine practice, as complete food intake by the child is unlikely.
Introduction
Temozolomide (TMZ) is an alkylating agent with demonstrated schedule-dependent clinical activity against malignant gliomas such as glioblastoma multiforme or anaplastic astrocytoma in patients from three years of age [1,2]. TMZ is also widely used as standard chemotherapy to treat pediatric cancers, including neuroblastoma [3,4,5], medulloblastoma [6], and rhabdomyosarcoma [7]. TMZ development was driven by the increasing interest in the delivery of anticancer drugs via the oral route, which is more convenient for self-administration and improves patients’ quality of life. Solid dosage forms, i.e., tablets and capsules, are the most preferred pharmaceutical forms because of their advantages, such as high dosing accuracy, easy handling, long-term stability in ambient conditions, and patient compliance. As TMZ dosing is based on the body surface area (BSA), the drug is currently supplied as hard capsules of six different strengths (Temodal®: 5 mg, 20 mg, 100 mg, 140 mg, 180 mg, and 250 mg) to enable dosing flexibility [2].
Temozolomide demonstrated rapid (Tmax ~1 h) and complete absorption (100% bioavailability) and a linear-to-dose pharmacokinetic (i.e., total body clearance) after oral administration of capsules in adult patients under fasting conditions [1,2]. However, the food effect specifically studied in a pharmacokinetic study in adult patients showed a significant impact on the pharmacokinetic profile with a Tmax increase and Cmax and AUC0–24 decrease [1]. As it cannot be excluded that the change in Cmax is clinically significant, current product guidance recommends that TMZ capsules must be swallowed whole with water on an empty stomach [2]. Nevertheless, the TMZ capsules are large (from approximately 16 mm (size 3 for TMZ 5 mg) to 22 mm (size 0 for TMZ 140 mg to 250 mg) in length), making it difficult for pediatric patients to swallow [8]. Even if the age at which children can swallow intact tablets or capsules is highly dependent on the individual and the training that they receive from healthcare professionals or caregivers, the size of tablets and capsules should be kept as small as possible [9] and less than 10 mm [10] to improve the acceptability. Furthermore, the administration of several capsules may be required to achieve the prescribed dose, which represents an additional barrier for children who are averse to swallowing the solid dosage form [11]. For TMZ treatment, according to the available dosage strengths, the common dose of 90 mg (corresponding to a child under the age of 6 exhibiting a BSA of 0.6 m2 and treated with 150 mg/m2 of TMZ) is achieved with four capsules of 20 mg and two capsules of 5 mg, i.e., six capsules of size 2 or 3 to be swallowed by the child. The combination of size and number of hard capsules of TMZ can affect oral acceptability and potential adherence to chemotherapy treatment.
In these cases, clinicians may often instruct caregivers to follow common administration practices in pediatrics [12,13] by opening the TMZ capsules and mixing the powder content into liquid and/or soft food before spoon-feeding or syringing the mixture to the child with the aim of improving swallowability and palatability [14,15]. Despite the lack of scientific information regarding the adverse effects associated with such drug manipulations or modifications [16], mixing medicines into foodstuffs to facilitate drug administration is frequent and occurs in one-third of medicines for oral administration to the pediatric population [11,17]. However, unless the impact of liquid or soft foods used as vehicles for drug administration on the drug product performance (i.e., drug product quality attributes) is appropriately assessed and justified [18], this approach should be avoided for several reasons. Firstly, TMZ is a cytotoxic and teratogenic alkylating agent, meaning that exposure to the powder can be harmful to caregivers and the family [8,15]. Secondly, administering the accurate dose may be inconsistent and compromised by incomplete food consumption by the child. Indeed, TMZ is a bitter substance and drug loss spilled, regurgitated, or left behind in the glass/pot is frequent. Manipulation and opening of the capsules may further increase the loss of TMZ (e.g., some powder left in the capsule shell) and thereby increase errors in accurate dosing. Third, as TMZ is unstable under light and at a pH of 7 or above, mixing the capsule contents with food may result in drug degradation and suboptimal dosing [15]. Finally, the co-administration of capsules with food may impact the bioavailability of TMZ [1] with the potential risk of subtherapeutic drug levels.
Considering these challenges, the European Medicines Agency (EMA) ‘Draft Inventory of Paediatric Therapeutic Needs’ highlighted the need for an age-appropriate formulation of TMZ [19].
Ped-TMZ (KIZFIZO®, temozolomide 40 mg/mL oral suspension) is a ready-to-use oral liquid pediatric formulation of TMZ that is currently in development for the treatment of relapsed or refractory neuroblastoma. This age-adapted and taste-masked formulation is designed to deliver an accurate high drug load while avoiding drug manipulation and caregiver exposure to TMZ. The objective of this study was to evaluate the impact of TMZ capsule manipulation on the main critical quality attributes, accuracy (assay), and stability of TMZ, compared with the ready-to-use Ped-TMZ suspension. Pediatric patients who require TMZ treatment typically receive doses of 80–120 mg [15]. For the purpose of this study, a dose of 90 mg was chosen corresponding to a child under the age of 6 exhibiting a BSA of 0.6 m2 and treated with 150 mg/m2 of TMZ. Different liquids and soft foods were selected with regard to the reported use [20] or likelihood to be used in the targeted pediatric population (children from the age of 1 year) and to cover the range of various food components (e.g., caloric content), physicochemical properties (e.g., pH, texture) and temperature administration (e.g., ambient or hot). Due to the pH-dependent hydrolysis of TMZ [21], vehicles of various pH levels were selected. Apple sauce and apple juice were selected for their acidic pH environment. The other vehicles (chocolate cream, mashed, and infant milk) were chosen for their anticipated neutral pH and their different textures, compositions, and temperature administrations. Additionally, apple sauce and apple juice (in which the chemical stability of TMZ is supposed to be favorable) were used to assess the potential impact of the vehicle texture (liquid vs. soft food) on the delivered dose, e.g., related to any potential loss of the vehicle. The holding time was assessed over one hour after the preparation of the mixture and was considered a reasonable timeframe consistent with real life.
Download the full article as PDF here Dispensing Oral Temozolomide in Children
or read it here
Lemarchand, C.; Bienaymé, H.; Rieutord, A.; Abbou, S.; Annereau, M.; Bastid, J. Dispensing Oral Temozolomide in Children: Precision and Stability of a Novel and Ready to Use Liquid Formulation in Comparison with Capsule Derived Mixtures. Pharmaceutics 2023, 15, 2711. https://doi.org/10.3390/pharmaceutics15122711
Read more on Orally Disintegrating Tablets (ODTs) here:


