Formulation, Preparation, Characterization, and Evaluation of Dicarboxylic Ionic Liquid Donepezil Transdermal Patches
Abstract
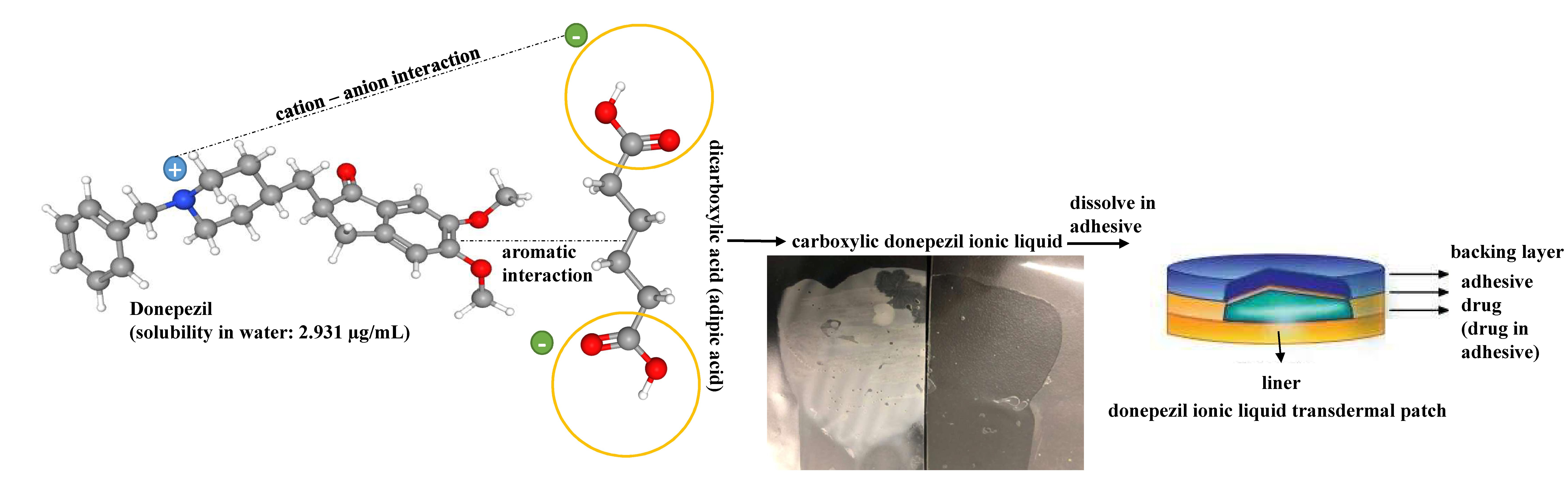
Donepezil (DPZ) is generally administered orally to treat Alzheimer’s disease (AD). However, oral administration can cause gastrointestinal side effects. Therefore, to enhance compliance, a new way to deliver DPZ from transdermal patch was developed. Ionic bonds were created by dissolving dicarboxylic acid and DPZ in ethanol, resulting in a stable ionic liquid (IL) state. The synthesized ILs were characterized by differential scanning calorimetry, optical microscope, Fourier transform infrared spectroscopy and nuclear magnetic resonance spectroscopy. The DPZ ILs were then transformed to a suitable drug-in-adhesive patch for transdermal delivery of DPZ. The novel DPZ ILs patch inhibits crystallization of the IL, indicating coherent design. Moreover, DPZ ILs and DPZ IL patch formulations performed excellent skin permeability compared to that of the DPZ free-base patch in both in vitro and ex vivo skin permeability studies.
Introduction
Alzheimer’s disease (AD), which has affected over 40 million elderly people, is the most common cause of dementia and one of the leading causes of death in vulnerable patients [1]. However, the mechanism underlying the occurrence of AD is not fully understood, and there is currently no cure for this disease, thus patients with AD become a considerable burden on their families and society [1,2]. The most prescribed agents that can temporarily manage dementia are cholinesterase inhibitors (ChEIs) such as donepezil, galantamine, and rivastigmine [3]. Among these ChEIs, donepezil (DPZ) has been reported to show beneficial effects in patients with more severe AD [4]. DPZ is generally administered orally in the treatment of AD [5]. However, oral administration can cause gastrointestinal side effects such as diarrhea, nausea, and vomiting due to drastic changes in plasma concentration upon oral administration [5]. In particular, most patients with AD refuse to take drugs or have difficulty in swallowing or chewing drugs due to dysphagia. Since the oral DPZ therapy is associated with adverse events and plasma concentration fluctuations, DPZ transdermal patch has been employed as an alternative route of administration. Traditional DPZ transdermal preparations contain many permeation enhancers owing to its low skin permeability, which may cause skin irritation problems; moreover, solid crystals are generated during storage, resulting in reduce adhesion and a non-uniform skin permeation rate. In this study, we developed a DPZ ionic liquid (IL) transdermal patch—a novel transdermal preparation of DPZ that can overcome the current drawbacks.
DPZ is an acetylcholinesterase inhibitor used to treat cognitive symptoms in mild to moderate AD [3,4,5]. In particular, it appears to benefit both cognitive ability and mental function [4,5]. DPZ, 2-[(1-benzyl-4-piperidyl) methyl]-5,6-dimethoxy-2,3-dihydroinden-1-one, is a piperidine-based agent that is chemically unique among all ChEIs. DPZ has been reported to be superior to others because of its high potency and selectivity for acetylcholinesterase in the central nervous system [5]. DPZ reversibly inhibits acetylcholinesterase and alleviates the symptoms of AD by increasing the concentration of acetylcholine and improving choline function. However, due to its low solubility (<0.1 mg/mL (insoluble) in H2O), DPZ still has limited applications as a potential therapeutic agent. Being a small molecular weight (179.31 g/mol (free base) and lipophilic (log p value of 3.08–4.11), DPZ has been considered to be a well-suited active pharmaceutical ingredient for transdermal delivery.
Transdermal drug delivery systems (TDDS) have the advantages of being non-invasive, self-administered, and having long-term drug action thus reducing dosing frequency. TDDS helps improve both patients’ and caregivers’ compliance compared to the parenteral and oral administration, especially in old patients. Moreover, TDDS provides a stable, uniform drug blood levels resulting in reduced side effects. Patches also permit rapid termination of drug delivery by stripping in case of any severe side effect experiences or signs of an overdose.
Several studies have shown ILs as promising active pharmaceutical ingredients in the development of transdermal formulation [6,7,8,9]. ILs have large scale industrial applications in this field over the past few years. ILs can bypass the barrier properties of the outermost layer of the epidermis and diffuse intracellularly through mechanisms such as disruption and fluidization of cellular integrity, generation diffusion pathways and extraction of lipid components of the stratum corneum [10,11]. An IL is defined as an organic salt in its liquid state [12]. The stable liquid phase is maintained over a wide range of low to high temperature [13]. ILs can be classified into different categories based on their thermodynamic and physicochemical properties: e.g., high-temperature molten salt, network-forming IL, low-temperature molten salt, and room temperature IL. In this study, we focused on room temperature ILs with melting point below 100 °C but not necessarily above 0 °C. Room temperature ILs typically contain large organic cations with basic cyclic structure and/or quaternized nitrogen and/or phosphorus atoms with attached alkyl chains.
Similar to most other active pharmaceutical ingredients, DPZ exists in a solid crystalline form. The most obvious problem with crystalline form is polymorphism. Polymorphism can negatively affect solubility, and ultimately the absorption and bioavailability of the drug [9]. In this study, DPZ was solubilized with several counterions (dicarboxylic acid) in ethanol (the solvent) and eventually converted to ILs. The aim of this study was to create a room temperature IL of DPZ (DPZ solubilizing form) that can suppress crystal formation for a prolonged duration to facilitate the sustained release of DPZ from a transdermal patch.
Download the full research paper as PDF: Formulation, Preparation, Characterization, and Evaluation of Dicarboxylic Ionic Liquid Donepezil Transdermal Patches
Materials
DPZ free-base was purchased from Perrigo (Allegan, MI, USA). Adipic acid, glutaric acid, isophthalic acid, itaconic acid, phthalic acid, saccharic acid, sebacic acid, terephthalic acid, and α-ketoglutaric acid were purchased from Deajung Chemicals and Metals (Siheung, Korea). Fumaric acid was purchased from Sigma Aldrich (St. Louis, MO, USA). Azelaic acid and pimelic acid were purchased from Alfa Aesar (Haverhill, MA, USA). Maleic acid and malic acid were purchased from Junsei Chemicals (Tokyo, Japan). Succinic acid was purchased from Samchun Pure Chemicals (Gyeonggi, Korea).
Ethanol and polyethylene glycol (PEG) 400 was purchased from Samchun Chemicals (Gyeonggi, Korea). Tween 80 was purchased from TCI Chemicals (Tokyo, Japan). Water was purified using Milli-Q® Reference water purification system (Merck Millipore, Alsace, France). DURO-TAK® 87-2051, DURO-TAK® 87-2074, and DURO-TAK® 87-2196 were provided by Henkel (Dusseldorf, Germany). Chloroform-D (D, 99.8%) containing 0.05% v/v tetramethylsilane was obtained from Cambridge Isotope Laboratories (Andover, MA, USA).
Female SKH1 hairless mice for animal studies were purchased from YoungBio (Seongnam, Korea). The mice were housed in standard cages placed in a semi-specific pathogen-free facility at 19 ± 1 °C and 50 ± 5% relative humidity, under a 12-h light–dark cycle. The mice were allowed to access to food and water freely prior to the experiments. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at Yonsei University, Seoul, Korea, and were performed according to the IACUC guidelines.
Ethanol and polyethylene glycol (PEG) 400 was purchased from Samchun Chemicals (Gyeonggi, Korea). Tween 80 was purchased from TCI Chemicals (Tokyo, Japan). Water was purified using Milli-Q® Reference water purification system (Merck Millipore, Alsace, France). DURO-TAK® 87-2051, DURO-TAK® 87-2074, and DURO-TAK® 87-2196 were provided by Henkel (Dusseldorf, Germany). Chloroform-D (D, 99.8%) containing 0.05% v/v tetramethylsilane was obtained from Cambridge Isotope Laboratories (Andover, MA, USA).
Female SKH1 hairless mice for animal studies were purchased from YoungBio (Seongnam, Korea). The mice were housed in standard cages placed in a semi-specific pathogen-free facility at 19 ± 1 °C and 50 ± 5% relative humidity, under a 12-h light–dark cycle. The mice were allowed to access to food and water freely prior to the experiments. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at Yonsei University, Seoul, Korea, and were performed according to the IACUC guidelines.
Dinh, L.; Lee, S.; Abuzar, S.M.; Park, H.; Hwang, S.-J. Formulation, Preparation, Characterization, and Evaluation of Dicarboxylic Ionic Liquid Donepezil Transdermal Patches. Pharmaceutics 2022, 14, 205. https://doi.org/10.3390/pharmaceutics14010205

