Development and evaluation of a composite dosage form containing desmopressin acetate for buccal administration
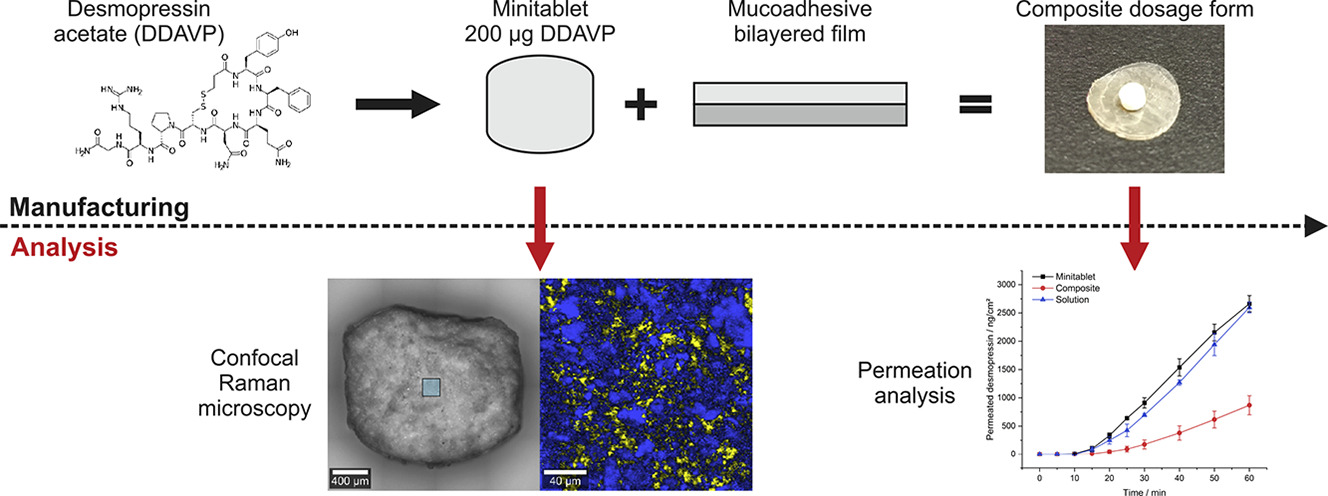
Desmopressin acetate (DDAVP) is an oligopeptide indicated for the treatment of primary nocturnal enuresis, for example. The poor oral bioavailability of DDAVP accelerated a shift to alternative routes of administration like nasal and oromucosal, whereby nasal administration results in high fluctuations increasing the risk of undesirable side effects.
Aim of the study was to use a new composite dosage form (solid matrix attached to a bilayer mucoadhesive film) to make DDAVP available via oromucosal route, reducing the risk of undesirable side effects through precise dosing. DDAVP was incorporated into a solid matrix in the form of a minitablet, and both direct tableting (AV > 30) and granulation followed by tableting (AV = 17.86) were compared. Minitablets with content uniformity could only be obtained by granulation and loss supplementation (AV = 11.27) with immediate drug release (>80% after 7–8 min) and rapid disintegration (<49 s). Permeation studies were performed with a clinically relevant dose (200 μg) in a time interval of up to one hour, resulting in apparent permeation coefficients of 4.90 × 10−6 cm/s (minitablet) and 2.04 × 10−6 cm/s (composite).
Comparable fluctuations showed no inferiority of composite and minitablet regarding dosing accuracy. Thus, a step towards controlled and dose-accurate transmucosal delivery of systemically active DDAVP could be achieved.
Download the full article as a PDF here or read it here
Materials: In this study, desmopressin acetate (PolyPeptide laboratories, Limhamn, Sweden) was used as the active pharmaceutical ingredient.
For the preparation of the MTs, α-lactose monohydrate (Flowlac 100, Meggle, Wasserburg, Germany), magnesium stearate (Parteck LUB MST, Merck, Frankfurt a.M., Germany) and fumed silicon dioxide (Aerosil 200, Evonik, Darmstadt, Germany) were used. The binder used for granulation was povidone 30 (Kollidon® 30, BASF, Ludwigshafen, Germany).
Chitosan (85% deacylated, Sigma Aldrich, Darmstadt, Germany), hypromellose (HPMC, Pharmacoat 603, Shin Etsu, Tokyo, Japan), and ethyl cellulose (EC, Aqualon N 22, Ashland, Kentucky, USA) were chosen as film-forming agents. Glycerol, at 85% (Caelo, Hilden, Germany), and triethylcitrate (Caelo, Hilden, Germany) were selected as plasticizers. Carmellose-natrium (CMC Na), at 20% (Walocel C 30 PA 09, Dow Wolff Cellulosics, Bomlitz, Germany) in aqueous solution was used as the glue between the MT and the MBF.
Na), at 20% (Walocel C 30 PA 09, Dow Wolff Cellulosics, Bomlitz, Germany) in aqueous solution was used as the glue between the MT and the MBF.
Water [high-performance liquid chromatography (HPLC)-grade] was obtained from Fisher Scientific (Hampton, USA). Acetonitrile (HPLC grade), ortho-phosphoric acid (85%, p.a.), and formic acid (98%, p.a.) were purchased from AppliChem (Darmstadt, Germany). Ethanol (99%), hydrochloric acid (0.1 N), and dipotassium hydrogen phosphate were provided by VWR Chemicals (Langenfeld, Germany). The buffer composition used in these studies contained 10 mM dipotassium hydrogen phosphate in distilled water, and the pH value (pH 6.8 or 7.4) was adjusted with orthophosphoric acid.
Biomimetic membrane systems by PermeaPad® (innoME, Espelkamp, Germany) were used as barriers for permeation measurements.
Article information: Dina Kottke, Bjoern B. Burckhardt, Tanja C. Knaab, Jörg Breitkreutz, Björn Fischer. Development and evaluation of a composite dosage form containing desmopressin acetate for buccal administration,
International Journal of Pharmaceutics: X, 2021. ://doi.org/10.1016/j.ijpx.2021.100082.

