Effects of omitting titanium dioxide from the film coating of a pharmaceutical tablet
An industrial case study of attempting to comply with EU regulation 2022/63
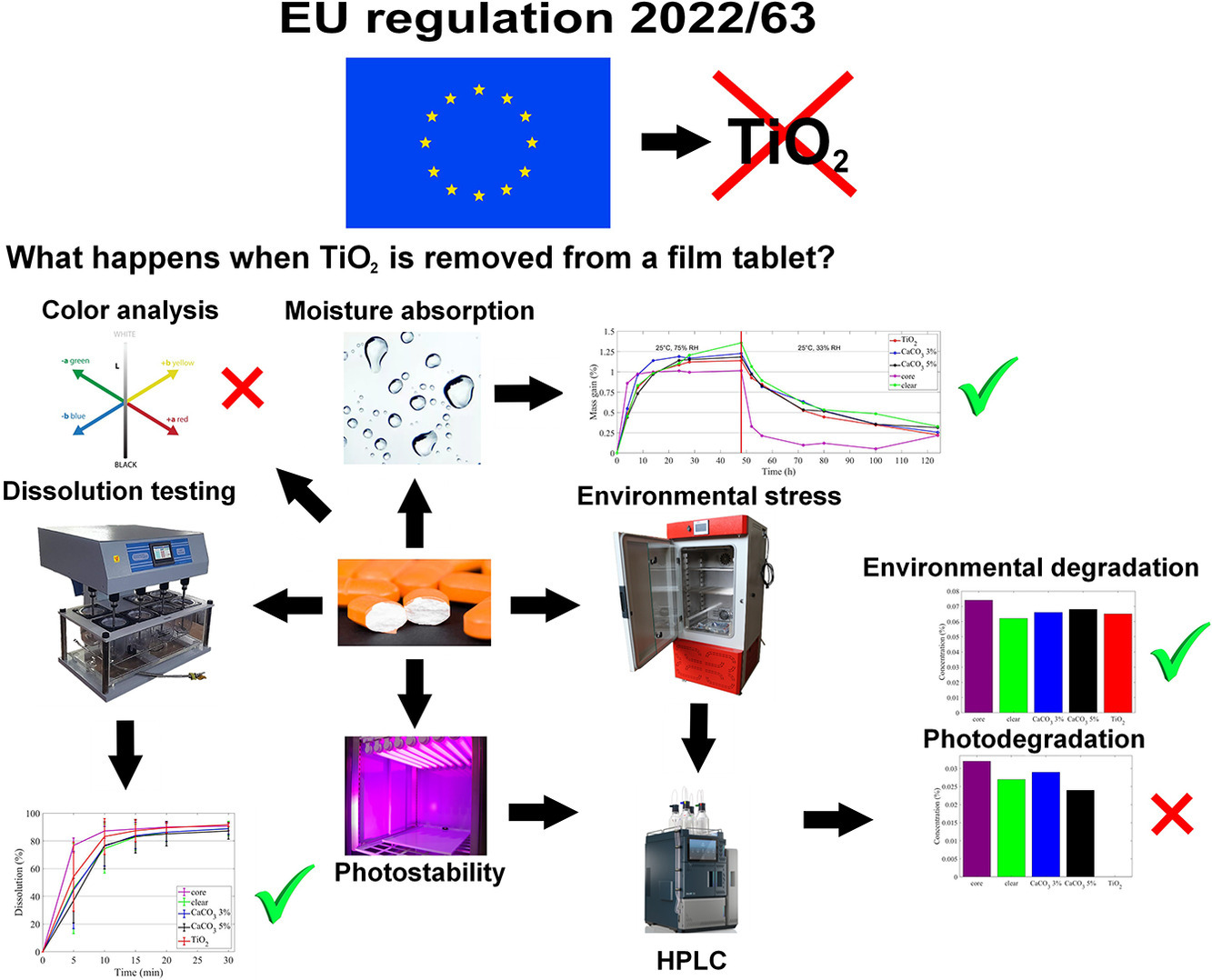
Recently, concerns have been raised about the safety of titanium dioxide (TiO2), a commonly used component of pharmaceutical film coatings. The European Union has recently prohibited the application of this material in the food industry, and it is anticipated that the same will happen in the pharmaceutical industry. For this reason, pharmaceutical manufacturers have to consider the possible impact of removing TiO2 from the film coating of tablets. In this paper, we present a case study of a commercially produced tablet where the film coating containing TiO2 was replaced with a coating using calcium carbonate (CaCO3) or with a transparent coating. The performance of the coatings was compared by measuring the moisture absorption rate and the dissolution profile of the tablets.
In these regards, there were negligible differences between the coating types. The tablets contained a highly photosensitive drug, the ability of the coatings to protect the drug was evaluated through environmental stability and photostability measurements. The HPLC results showed that the inclusion of TiO2 does not provide additional benefits, when humidity and thermal stress is applied, however its role was vital in protecting the drug from external light. There were several decomposition products which appeared in large quantities when TiO2 was missing from the coating. These results imply that photosensitivity is an issue, replacing TiO2 will be challenging, though its absence can be tolerated when the drug does not need to be protected from light.
Download the full article as PDF here Effects of omitting titanium dioxide from the film coating of a pharmaceutical tablet
or read it here
Materials
2.1 Film coating of tablets
This study was performed on a commercial film coated pharmaceutical tablet formulation, which composition is confidential. The formulation was selected for the study, because the API is known to have high photosensitivity. The tablets contain microcrystalline cellulose, lactose monohydrate, crospovidone, magnesium hydroxide and magnesium stearate as excipients. The tablet cores had a mass of 300 mg and a diameter of 9 mm. The friability of the tablet cores was measured by placing approximately 6.5 g of tablet cores in the drum of a Pharmatest PTF 10E friability tester (Pharma Test Apparatebau, Hainburg, Germany), then they were rotated 100 times. This test was repeated 3 times. The friability was found to be 0.14 % ± 0.02 %.
The tablet cores were coated using 3 different products, which exact type is confidential. All 3 use poly(vinyl-alcohol) (PVA) as polymer, the first contains TiO2 as opacifier in a concentration of 25 w/w%. The second utilized CaCO3 as an alternative opacifier in 25 w/w%, while the third was a clear coating. In order to study how TiO2 performs compared to the alternatives, 4 different formulations were prepared, where one of the formulations is tested with two different coating levels, resulting in a total of 5 tested formulations. As a reference, the uncoated tablet cores were also examined (henceforth referred to as ‘core’). PVA-based coatings were chosen exclusively because we intended to simulate a scenario where the manufacturer needs to omit TiO2 from the coating, in this case other parameters of the technology need to remain as similar as possible. For this reason, changing the coating to a hydroxypropyl-methylcellulose (HPMC)-based product would be considered a more drastic alteration of the technology. The coating containing TiO2 was applied for a weight gain of 3 % (‘TiO2’). The coating with CaCO3 was applied with 3 and 5 % weight gain to determine whether a thicker coating might offset the anticipated weaker performance of CaCO3 in protection from light, these formulations will be called ‘CaCO3 3 %’ and ‘CaCO3 5 %’, respectively. The clear coating was applied with 3 % weight gain (‘clear’). The weight gains of 3 % and 5 % are equivalent to 6.1 mg/cm2 and 10.1 mg/cm2 of coating weight per unit of tablet surface area, respectively.
Film coating was performed on batches of 800 g tablets in a Glatt GB2 L50-10026 pan coating machine (Pratteln, Switzerland). Table 1 contains the parameters used during the process. After film coating, the tablets were stored for 10 days in plastic bags inside a container that protected them from light at 25 °C and 30 % relative humidity before the measurements described below were carried out.
Dorián László Galata, Melinda Sinka Lázárné, Dorottya Kiss-Kovács, Gergő Fülöp, Barnabás Dávid, Botond Bogáti, Máté Ficzere, Orsolya Péterfi, Brigitta Nagy, György Marosi, Zsombor Kristóf Nagy, Effects of omitting titanium dioxide from the film coating of a pharmaceutical tablet – An industrial case study of attempting to comply with EU regulation 2022/63, European Journal of Pharmaceutical Sciences, Volume 196, 2024, 106750, ISSN 0928-0987, https://doi.org/10.1016/j.ejps.2024.106750.
See our next webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


