Freeze-Drying of Encapsulated Bacteriophage T4 to Obtain Shelf-Stable Dry Preparations for Oral Application
Therapeutic application of bacterial viruses (phage therapy) has in recent years been rediscovered by many scientists, as a method which may potentially replace conventional antibacterial strategies. However, one of the main problems related to phage application is the stability of bacterial viruses. Though many techniques have been used to sustain phage activity, novel tools are needed to allow long-term phage storage and application in versatile forms. In this study, we combined two well-known methods for bacteriophage immobilization.
First, encapsulated phages were obtained by means of extrusion–ionic gelation, and then alginate microspheres were dried using the lyophilization process (freeze-drying). To overcome the risk of phage instability upon dehydration, the microspheres were prepared with the addition of 0.3 M mannitol. Bacteriophage-loaded microspheres were stored at room temperature for 30 days and subsequently exposed to simulated gastric fluid (SGF). The survival of encapsulated phages after drying was significantly higher in the presence of mannitol.
The highest number of viable bacteriophages exceeding 4.8 log10 pfu/mL in SGF were recovered from encapsulated and freeze-dried microspheres, while phages in lyophilized lysate were completely inactivated. Although the method requires optimization, it may be a promising approach for the immobilization of bacteriophages in terms of practical application.
1. Introduction
Antimicrobial resistance of bacteria has in recent decades become a worsening global problem. In light of the limited efficacy of frontline antibiotics and the decreasing number of new antibacterial drugs introduced onto the market each year, innovative antibacterial strategies are urgently needed. In this context, the application of bacteriophages may be a promising approach.
Research in recent years has shown that using different techniques of immobilization may significantly improve phage stability in the stomach environment as well as during storage [13,14]. However, the golden mean is needed—a method which would provide broadly defined phage stability and at the same time a formulation which is convenient and patient-friendly.
The objective of the study was to develop a method for obtaining a phage preparation in the form of a dry powder, making it possible to retain phage viability during storage and subsequently in an acidic stomach environment. We have combined two well-known techniques for phage immobilization. In the first stage, we encapsulated phages with an extrusion method. Then, to obtain the dry formulation, we used lyophilization (freeze-drying), a technique which is frequently used for the immobilization of molecules and microorganisms [14]. Since the dehydration of bacteriophage preparations may relate to significant titer loss, at the encapsulation stage, we used mannitol as an excipient, the protective properties of which we have demonstrated in previous studies [15]. Although the proposed method requires further optimization, we believe it may be a promising approach for the immobilization of bacteriophages in terms of practical application.
…
2.2. Encapsulation of Bacteriophage T4
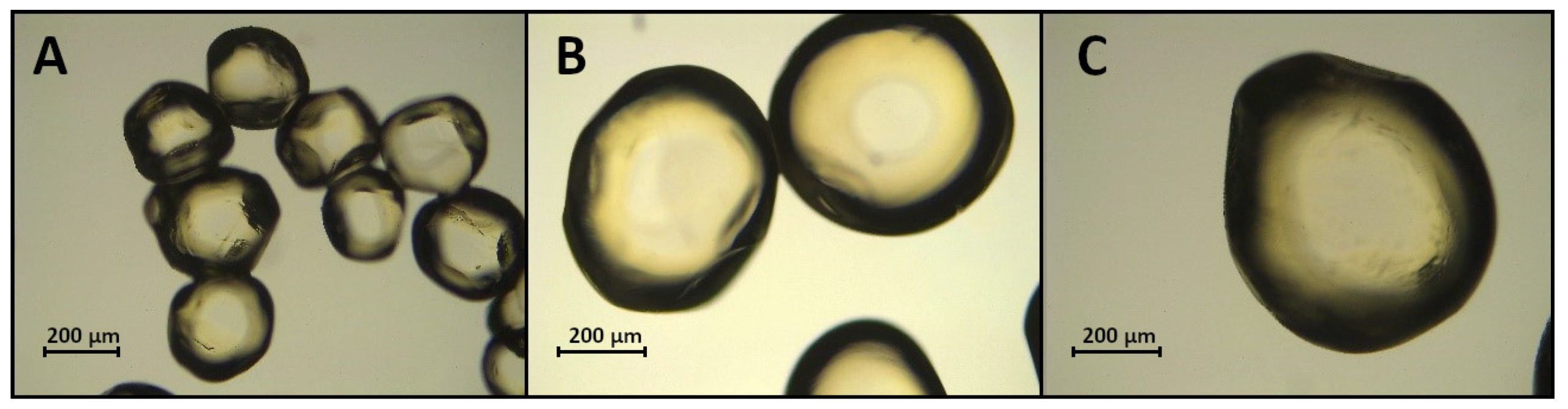
Ca-alginate microspheres were prepared using an extrusion method adapted from [15] and modified. Bacteriophages were encapsulated in alginate (Sigma-Aldrich, Darmstadt, Germany) or alginate with mannitol (Sigma Aldrich, Germany) capsules using encapsulator B-390 (Büchi Labortechnik AG, Flawil, Switzerland). Some 30 mL of T4 lysate was mixed with 90 mL of 2% water solution of sodium alginate. Then, the mixture was extruded through the nozzle with a diameter of 150 µm, 300 µm or 450 µm into the 0.1 M CaCl2 or 0.1 M CaCl2 supplemented with 0.3 M mannitol at 23 °C. The following parameters of the encapsulation process were used: air pressure 150 mbar, frequency 800 Hz, and stream height 30 cm. The beads were kept in the solution for 20 min and were allowed to harden. Then, the formed beads were washed with distilled water. The shape and diameter of the capsules were investigated under the light microscope (Zeiss Axio Vert.A1 for wet microspheres and Leica DMI1 for dry microspheres).
Download the full study as PDF here: Freeze-Drying of Encapsulated Bacteriophage T4 to Obtain Shelf-Stable Dry Preparations for Oral Application
or read it here
Śliwka, P.; Skaradziński, G.; Dusza, I.; Grzywacz, A.; Skaradzińska, A. Freeze-Drying of Encapsulated Bacteriophage T4 to Obtain Shelf-Stable Dry Preparations for Oral Application. Pharmaceutics 2023, 15, 2792.
https://doi.org/10.3390/pharmaceutics15122792

