Evaluation of Printability of PVA-Based Tablets from Powder and Assessment of Critical Rheological Parameters
Abstract
Introduction
Three-dimensional printing as part of additive manufacturing has had huge impacts on different parts of technological advancements. With the beginning of research on the use of 3D printing in medical and pharmaceutical disciplines, the hopes for using the benefits of these techniques have risen. With Spritam® and ZipDose® technology (Aprecia Pharmaceuticals, LLC, Blue Ash, OH, USA), the first 3D printed tablet, using a combination of powder layering and inkjet technology, was approved by the FDA in 2015 [1]. Different extrusion-based technologies such as semi-solid extrusion (SSE) [2,3] and fused deposition modeling (FDM) were considered in the research of medical devices and pharmaceutical products, so much so that recently Triastek (Triastek, Inc., Nanjing, China) has received IND (Investigational New Drug) clearance for their second 3D printed product T20 using melt extrusion deposition (MEDTM) technology [4]. With the rise of extrusion-ready pharmaceutical-grade excipients, tablets with different release patterns were produced using the FDM-based 3D printing technique. In terms of orally administered dosage forms, immediate release [5], modified release [6,7], bilayer [8], and even intra-gastric floating tablets [9,10] have been developed. The main advantage of 3D printed medicines is seen in the ability to produce customizable and patient-oriented geometries and strengths of tablets as well as in rapid prototyping [11]. While the general acceptability of 3D printing has been shown, concerns were found regarding different shapes. Already established shapes such as capsule or disc-shaped printlets are regarded as acceptable for swallowing, whereas more intricate shapes such as tilted diamonds were harder to “sell” to the testing group [7]. The first approaches of 3D printing used commercially available 3D printers and filaments. The filament was loaded with the API (active pharmaceutical ingredient) using different techniques such as loading by soaking [12]. The disadvantages found in the control of the loading and general concerns about solvent usage over time rendered this method obsolete. The next step was to extrude the filament needed directly from raw materials and to include the API in the desired concentration [13]. Filament production is challenging since an additional heating step is needed, which can negatively impact thermo-degradable drugs and polymers. Also, production must be strictly controlled to result in a sufficiently uniform filament. To avoid these problems, the extrusion and printing must take place in a single step. The company FabRx (FabRx Ltd., London, UK) developed a 3D printer especially for use in the development of pharmaceutical products. The filament printhead is exchanged for a printhead consisting of a small-scale single-screw extruder with a detachable nozzle which enables so-called direct powder extrusion (DPE) [14]. This opened the door to the printing of many of the excipients already developed for hot-melt extrusion (HME). While printing with filaments requires extensive knowledge of excipients and devices to produce filaments with relevant properties such as tensile strength, these parameters are not relevant when printing directly from the powder. Mechanical properties are key to printability for filament printers. A main prerequisite is longitudinal rigidity, which is shown by a high Young’s modulus while allowing no deformation or breakage during mechanical stress in printers feeding elements [15]. Thus, printing temperature can be reduced for the use of thermo-degradable drugs and polymers by incorporating plasticizers.
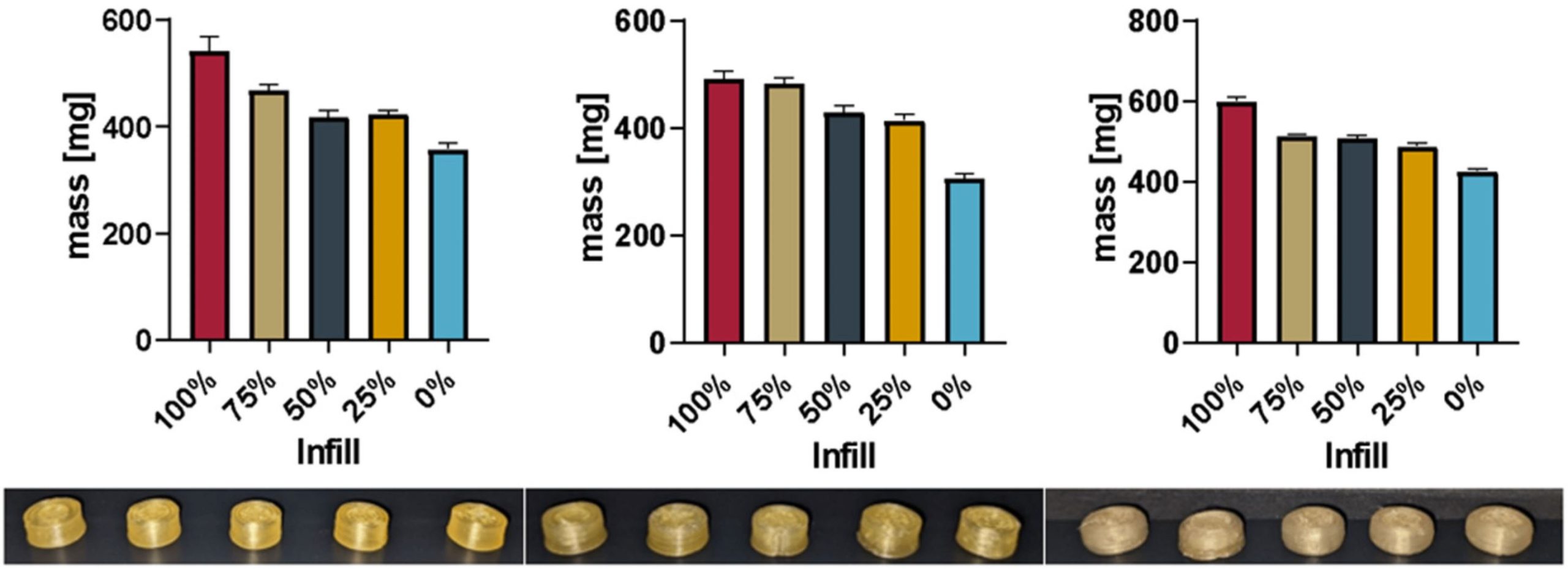
The aim of our research was to show that biopharmaceutics classification system (BCS) class II [16] drugs can be directly printed from powdered excipients into tablets with different strengths while forming amorphous solid dispersions (ASD). BCS class II compounds show low (water-) solubility and high permeability, so an improvement of the solubility is often searched for. Polyvinylalcohol (PVA) is a well-researched pharmaceutical excipient that shows appropriate thermal behavior (no thermal degradation up to at least 230 °C, appropriate melt viscosity) as well as water solubility [17]. As per the safety data sheet, the melting region is listed as 160–240 °C and the degradation temperature as above 200 °C. The synthetic opioid agonist loperamide (hydrochloride) [18] was used as a thermally stable BCS class II compound. One of the main challenges was to be able to form ASDs while performing the printing at temperatures well below the melting point of loperamide (220–228 °C [19]) since the thermal degradation of PVA starts below the melting temperature of loperamide. Information about the requirements of excipients for extrusion/3D printing using direct powder extrusion is scarce. Thus, a rheological model for PVA showing the minimum melt viscosity needed as well as the temperature and shear rate dependency was investigated.
Melt rheology and information about extrusion processes can be crucial for hot-melt extrusion as well as extrusion-based 3D printing. In previous studies, different approaches have been investigated to generate information regarding certain printers. Temperature as well as the closely related melt viscosity were parameters that were found to be of importance for further development [20,21]. Too low viscosity leads to oozing and dripping from the nozzle, reducing print quality, while too high viscosity leads to improper material flow up to a clogged nozzle or reduced layer adhesion.
Regarding the release behavior, the aim was set to be equivalent to typical formulations with immediate to sustained release.
Download the full article as PDF here: Evaluation of Printability of PVA-Based Tablets from Powder and Assessment of Critical Rheological Parameters
or read it here
Materials
PVA (Parteck® MXP (Polyvinylalcohol), Merck KGaA, Darmstadt, Germany) was used as a water-soluble pharma-grade excipient available for (HME). Fumed silica (Aerosil® R 972 Pharma, Evonik, Essen, Germany) was added to improve the flowability of the powder mixtures. As model API, loperamide hydrochloride (100% purity, Merck KGaA, Darmstadt, Germany) was selected. As a commonly used plasticizer for PVA, sorbitol (Parteck SI 400, Merck KGaA, Darmstadt, Germany) was used. All solvents used (Methanol, Acetonitrile, ammonium acetate) were HPLC-grade.
Lenhart, J.; Pöstges, F.; Wagner, K.G.; Lunter, D.J. Evaluation of Printability of PVA-Based Tablets from Powder and Assessment of Critical Rheological Parameters. Pharmaceutics 2024, 16, 553. https://doi.org/10.3390/pharmaceutics16040553
Vitafoods Geneva 2024 – with a focus on excipients


