Development of Extended-Release Formulation of Ibuprofen Using Blends of Calcium Silicate and Polyvinyl Pyrrolidone as Tablet Matrix
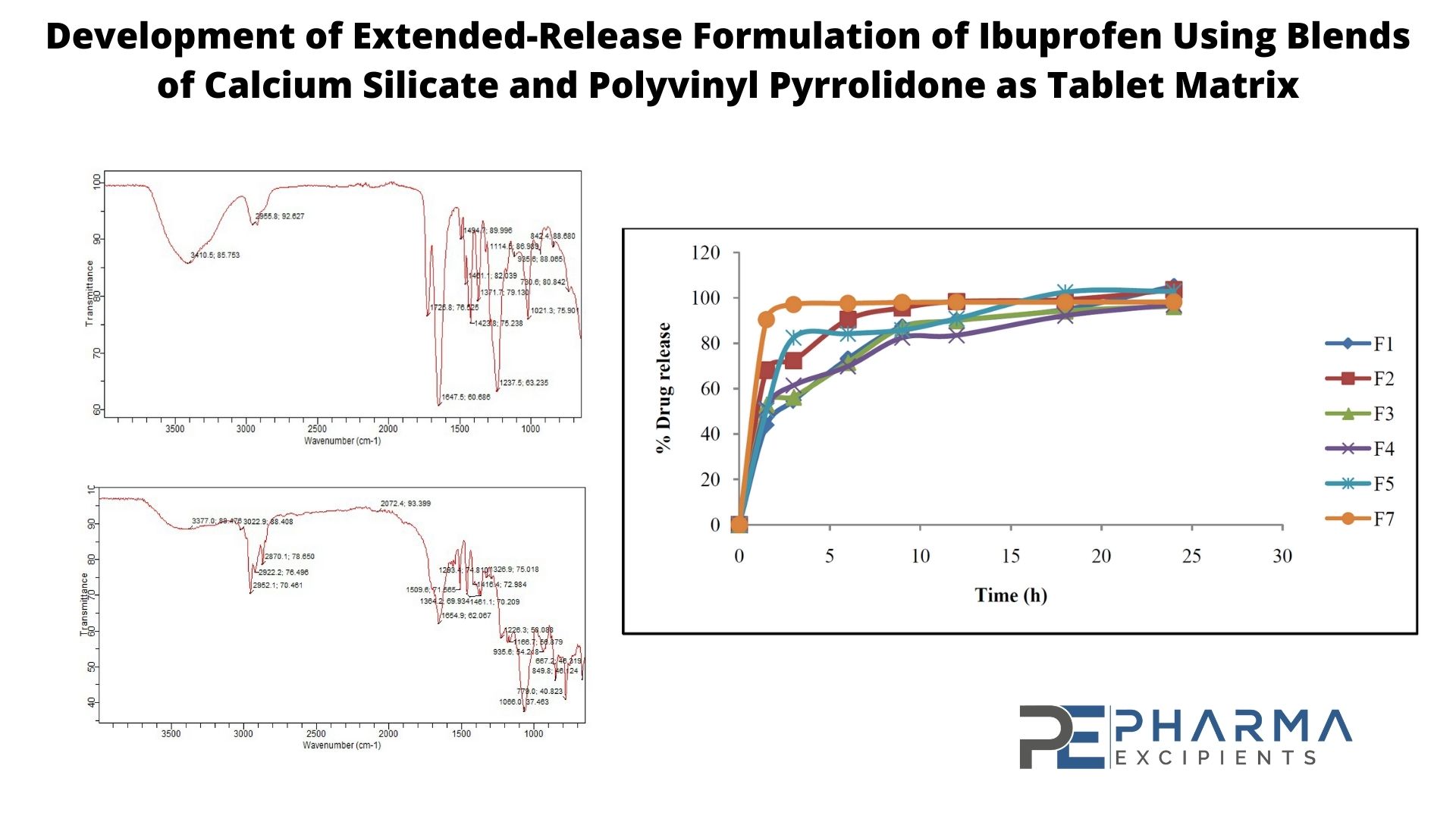
A tablet matrix system was developed for ibuprofen and the influence of the polymer blend and concentration on the release rate of the drug was evaluated. Tablets containing different concentrations of calcium silicate and PVP (polyvinyl pyrrolidone) were prepared using direct compression and the weight uniformity, crushing strength, friability, drug content uniformity, dissolution profile, and in vitro release kinetics were examined.
Formulated tablets were found to be within the official acceptable limits of physical and chemical parameters except for the thickness test that was below the conformation of extended-release tablets. The crushing strength of the tablets was in the range of 2.5 to 5.6 kg/f, the weight variations of the tablets of all the formulation was less than ± 5%. The friability of all the formulations was in the range of 0.6% to 1.83%. Tablet thickness and diameter was in range of 3.18 mm to 4.48 mm and 12.53 mm to 12.64 mm respectively.
Absolute drug contents of all the formulations were found to be in range of 83.50% to 98%. The release kinetic of F3 containing 20 mg of calcium silicate, 40 mg of PVP as matrix formers showed the best linearity (r2 = 0.6975) with % drug release of 96 showing that combination of the two polymers (20 mg calcium silicate and 40 mg PVP) for use as a matrix former is best for extended-release formulation of ibuprofen.
Download the full article as a PDF here or read it here
Article Information: Stephen Olaribigbe Majekodunmi and Margaret Ekong Dickson. J. Chem. Chem. Eng. 14 (2020) 119-128. doi: 10.17265/1934-7375/2020.04.003
Materials: PVP ( Kollidon VA 64 Fine), Lactose ( Granulac® 140 )

