Improving Active Pharmaceutical Ingredient Formulation by Hot Melt Extrusion

This poster was presented at PBP World Meeting 2024 in Vienna:
The integration of Artificial Intelligence (AI) and big data into pharmaceutical research over the past two decades has revolutionized drug discovery, enabling the identification of therapeutic targets with high accuracy and efficiency. (1) However, this progress introduces challenges, notably due to the non-polar nature and the chemical and physical properties of new active pharmaceutical ingredients (APIs), with about 90% classified under BCS classes II and IV, characterized by low water solubility and poor bioavailability. (2) Utilizing API modification techniques like Amorphous Solid Dispersions (ASD) which enhance solubility, bioavailability, and potentially reduce the drug dosage, thereby minimizing side effects and improving patient compliance. (3)
Hot Melt Extrusion Technology
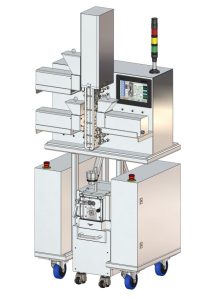
Hot Melt Extrusion (HME) is a solvent-free, low energy continuous process for pharmaceutical manufacturing, integrating multiple operations to efficiently produce products with APIs and excipients. This process heats and mixes materials in a barrel, forming a homogeneous mixture, enhancing safety and sustainability. The mixture is then shaped through a die, cooled and prepared for further processing. HME enhances stability and allows to reduce API dosing while maintaining the efficiency of treatments, reducing their secondary effects and lowering their cost. It also allows for diverse pharmaceuticals forms such as tablets, capsules, patches, films… promoting patient comfort and personalized medicine. (3-5)
Key HME parameters include barrel temperature, screw speed, design, and feed rate, crucial for preserving component integrity and ensuring final product quality.
Our HME system’s compact design maximizes space efficiency and ease of use, featuring materials resistant to extrusion processes for durability. Components in contact with products are designed for easy removal and cleaning, minimizing cross-contamination risk. A versatile screw design allows for precise shear and mixing adjustments, essential for optimal product characteristics. This design reduces operational and installation costs, offering an economical solution for R&D and manufacturing. (5)
Child Friendly ASD Formulation for Malaria
Malaria, a life-threatening disease caused by Plasmodium parasites and transmitted by female Anopheles mosquitoes, led to 242 million cases and an estimated 608,000 deaths in 2022, with children under 5 being particularly
vulnerable. Despite WHO’s global strategy initiated in 2015, aimed at reducing malaria incidence and death rates, especially among children, these targets remain unmet. Lumefantrine (LUM), a BCS class IV drug with poor aqueous
solubility and low absorption, is used in combination with Artemether (AT) as in Coartem®/Riamet® Dispersible tablets for treating uncomplicated malaria. This combination benefits from AT’s rapid action on parasitemia and LUM’s longer-lasting effects. However, LUM’s low oral bioavailability, influenced by dietary intake, poses challenges for effective treatment, potentially leading to treatment failure and malaria recurrence. (6) The collaborative project between Rondol, BASF and Queen’s University Belfast aim to develop and manufacture a robust child friendly fixed dose combination (FDC) while reducing the dose strength of Lumefantrine and the frequency of administration.
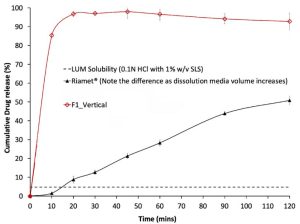
In-vitro drug dissolution profiles of F1 formulation (30% LUM 70% Soluplus). Dissolution tests were performed by placing formulations containing equivalent to 120 mg of LUM into 1 L of 0.1N HCl solution containing 1% (w/v) SLS. The dissolution profile of the Riamet, obtained using the same dissolution method, is plotted as comparison references. The dashed line represents the measured saturated solubility of crystalline LUM powder in the same medium. F1 resulted in higher release percentage : 84.8 ± 2.3% , compared to the commercial product 50.1 ± 1.4%.
Aspirin ASD for oncology applications
Acetylsalicylic acid (ASA), one of the most widely used medications globally, offers analgesic, antipyretic, and anti inflammatory properties. Beyond these applications, aspirin is also utilized as an antiplatelet agent, aiding in the prevention of heart attacks and strokes by inhibiting blood clot formation. Additionally, emerging evidence suggests that regular aspirin use may have a role in cancer prevention and treatment, particularly in reducing the risk of colorectal cancer and possibly other types. Nonetheless, daily consumption of aspirin carries the risk of several adverse effects. These include gastrointestinal issues like ulcers and bleeding due to aspirin’s action on the stomach lining, an increased risk of hemorrhagic stroke, and renal impairment with long-term use. (7) The collaborative project between Rondol and Seqens aims to develop and manufacture a formulation that enables the daily use of Aspirin in the field of oncology.
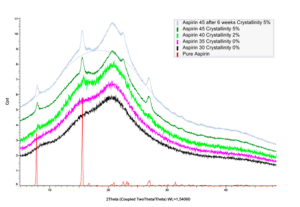
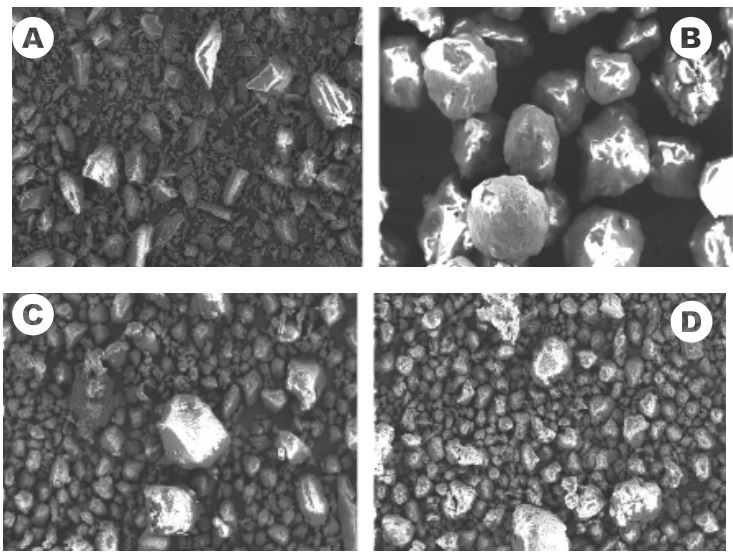
XRD diffraction pattern for 30, 35, 40 and 45 % w/w ASA-loaded pellets manufactured via HME with Soluplus at 130°C and pure ASA.
ASA can be fully amorphous at differents loadings and remains so more than 6 weeks after the manufacture which is promising for their longterm stability.
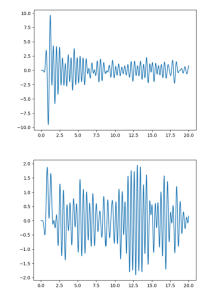
Pair distribution function (PDF) pattern for 30 % w/w ASA-loaded pellets manufactured via HME at 115°C with Soluplus and pattern for 30 % w/w ASA-loaded pellets manufactured via HME at 115°C with 5% Kollidon 12PF and 65% Soluplus.
Pair distribution function (PDF) analysis for ASD is a powerful technique used to gain insight into the short- and medium-range order of atoms or molecules within amorphous materials. By applying PDF analysis to ASDs, we can obtain detailed information on the arrangement and interaction of the API and the polymer matrix at a molecular level. This method helps in understanding the homogeneity of the dispersion, the extent of amorphousness, and the nature of API-polymer interactions that contribute to the stabilization and enhanced solubility of the API. (8) Additionally, Ultra Small Angle X-Ray Scattering (USAXS) will be utilized to delve into this topic, offering insights into the mechanisms and locations of interaction between the API and the polymer. (9
See the full technical poster on “Improving API Formulation by HME” here
(click the picture to download the poster)
Source: Maël Gallas, Victoire A de Margerie, Pascal Boulet, Ghouti Medjahdi, technical poster “Improving API Formulation by HME“, PBP World Meeting 2024