Understanding the Interaction of Thermal, Rheological, and Mechanical Parameters Critical for the Processability of Polyvinyl Alcohol-Based Systems during Hot Melt Extrusion
Abstract
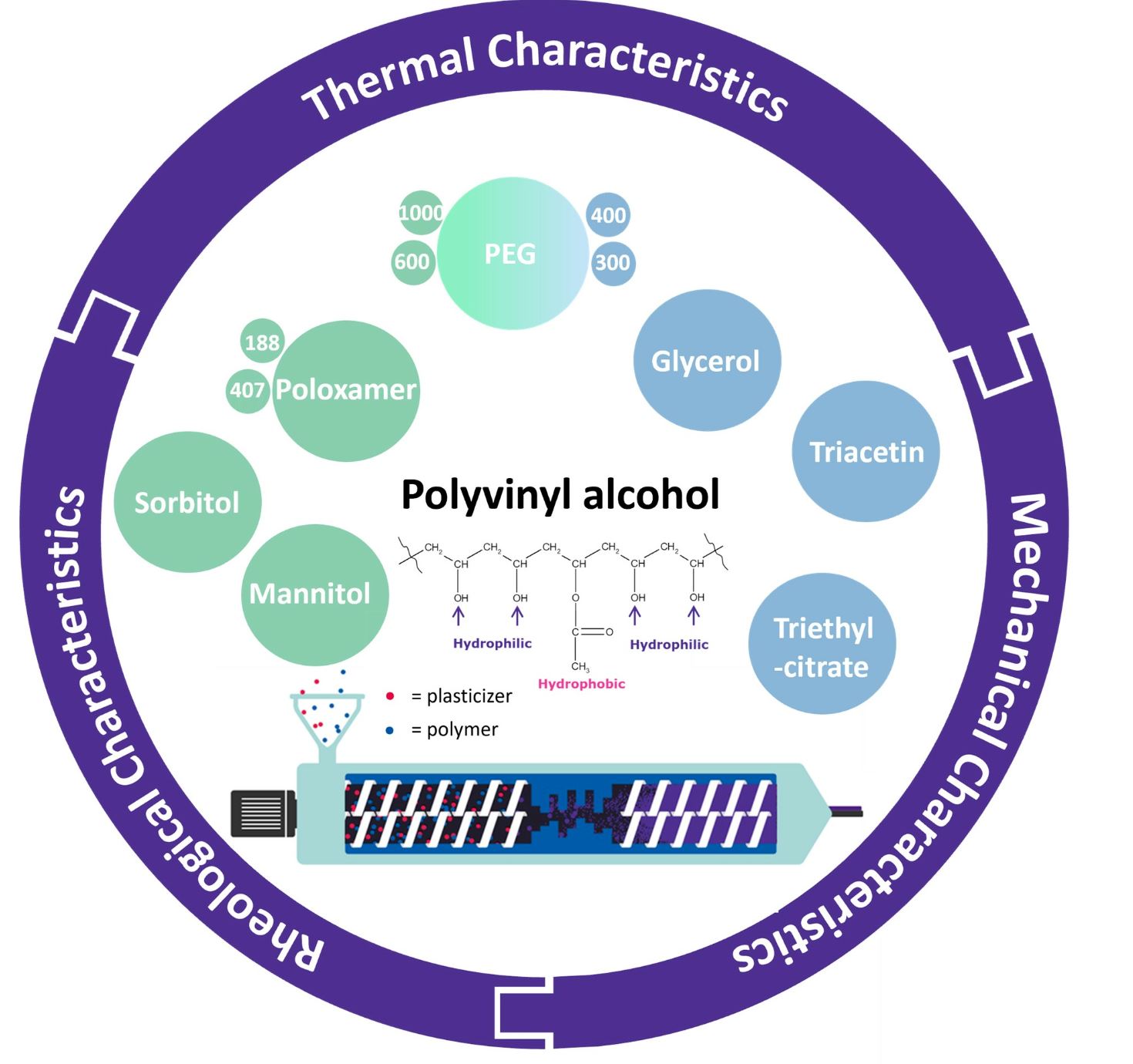
Hot melt extrusion (HME) is a common manufacturing process used in the pharmaceutical industry to improve the solubility of poorly soluble active pharmaceutical ingredients (API). The goal is to create an amorphous solid dispersion (ASD) where the amorphous form of the API is stabilized within a polymer matrix. Traditionally, the development of pharmaceutically approved polymers has focused on requirements such as thermal properties, solubility, drug–polymer interactions, and biocompatibility. The mechanical properties of the material have often been neglected in the design of new polymers. However, new downstream methods require more flexible polymers or suitable plasticizer polymer combinations. In this study, two grades of the polymer polyvinyl alcohol (PVA), which is already established for HME, are investigated in terms of their mechanical, rheological, and thermal properties. The mechanical properties of the extruded filaments were tested by the three-point bending test. The rheological behavior was analyzed by oscillating plate measurements. Thermal analysis was performed by differential scanning calorimetry (DSC). In addition, the solid and liquid plasticizers mannitol, sorbitol, triacetin, triethyl citrate, polyethylene glycol, and glycerol were evaluated for use with PVA and their impact on the polymer properties was elaborated. Finally, the effects of the plasticizers are compared to each other, and the correlations are analyzed statistically using principal component analysis (PCA). Thereby, a clear ranking of the plasticizer effects was established, and a deeper understanding of the polymer–plasticizer interactions was created.
Introduction
A large proportion of the active pharmaceutical ingredients (APIs) known today are poorly soluble in water, which poses challenges for the formulation of efficacious medicines [1]. Ting et al. [2] showed a breakdown of currently known APIs and pipeline substances in the Biopharmaceutical Classification System (BCS) [3]. A look at the distribution of APIs on the market, mentioned at the beginning, shows that 30% of the substances fall into BCS class II and 10% into BCS class IV. Looking at the current pipeline substances, i.e., the potential drug candidates, about 60–70% are BCS class II candidates, and an additional 10–20% are in BCS class IV [2]. The BCS class II candidates are of special interest. For them in particular, the solubility of the drug in biorelevant media is the decisive factor to act systemically and thus potentially be suitable as an oral form of administration [4,5].
To improve solubility, many methods are available [6]. The development of so-called amorphous solid dispersions (ASDs) is a particular focus in formulation development. In ASDs, the lattice structure of the API is opened up and the APIs are embedded in their amorphous state, for example, in a polymer matrix. This affects the drug solubility and dissolution rate, which can lead to an increased bioavailability [7,8,9,10]. For the production of amorphous solid dispersions (ASDs), various methods can be employed, such as spray drying [11], solvent evaporation [12], or high-energy milling [13]. Additionally, the supercritical fluid extraction technique has emerged as an alternative approach for the fabrication of ASDs, categorizing this method under adsorption on supports [14]. The other ASD production methods can be broadly classified into groups: solvent-based, fusion, or melting methods [15].
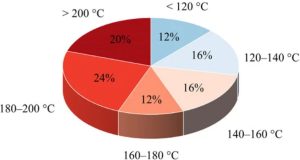
A well-established melt-based manufacturing process for ASDs is hot melt extrusion (HME) [16,17,18]. In this process, drug-excipient mixtures, often with polymers as a carrier matrix, are heated and mechanically mixed using one or more screws with special shear elements. The mixture becomes a viscous melt that is forced through a die. After cooling, the API remains dispersed in the polymer matrix, often in an amorphous state. This technique is already widely used in the pharmaceutical industry and several products are already on the market [19]. The APIs whose solubility could be improved by HME have different temperature-dependent process windows; these process windows must fit into the requirement profile of the polymer. The melting temperature (Tm) provides a preview of the temperature at which the APIs can be processed by HME. In Figure 1, the distribution of the melting points of the 24 APIs listed by Alzahrani et al. [19], which are processed via HME, is shown. Considering this broad distribution, it highlights how different temperature requirements must be met by suitable polymers in order to cover the variety of APIs.
In addition to the thermal properties, the development of pharmaceutically approved polymers has traditionally focused on requirements such as solubility, drug–polymer interactions, and biocompatibility. With the advent of new downstream methods such as strand pelletization, die-face pelletization, and film extrusion new mechanical demands were placed on the polymers [20]. This has been further emphasized with the emergence of 3D printing as a new technology in the pharmaceutical application field. Three-dimensional printing for pharmaceutical applications offers promising advantages, especially its high level of flexibility provides new approaches in the field of personalized medicine and in the acceleration of clinical trials. Therefore, there is increasing interest from pharmaceutical stakeholders to evaluate the different technologies and to develop products specifically designed for the different technologies [21]. Very prominent among the 3D printing technologies are the melt-based approaches, these include semisolid extrusion [22], direct powder extrusion [23], and fused deposition modeling (FDM) [24]. Melt-based 3D printing requires distinct polymer properties. Therefore, the focus lies in the identification of suitable polymers that have already been approved by medical regulatory authorities.
For extrusion-based 3D printing systems, thermoplastics are extruded layer upon layer to create the desired three-dimensional object. Analogous to the HME process, the temperature processing range of the APIs is a decisive factor for these technologies. Especially for FDM printing, there is a special requirement. In this technology, beyond the thermal requirements, another bottleneck is often the brittleness of the polymers, as filaments are re-melted prior to the printing process. The tight path to the print head and the conventional feed mechanism made of gears require a suitable mechanical stability of the polymer filaments used. A lack of mechanical stability can be compensated with printer modifications [25] or plasticizer combinations [26,27].
Some pharmaceutical approved polymers were already investigated for these technologies including celluloses such as ethyl cellulose [28], hydroxypropyl cellulose [24], hypromellose acetate succinate [29], vinylpyrrolidone-vinyl acetate copolymers [30], polymethacrylates [31], and polyvinyl caprolactam derivatives [32]. A polymer that is frequently applied for 3D printing is polyvinyl alcohol (PVA) in pharmaceutical grades [33].
PVA is used in many pharmaceutical applications and is a well-characterized polymer for the HME [34,35,36] process. PVA is nontoxic, biocompatible, and water soluble. Often-used grades for hot melt extrusion are PVA 3-82 and PVA 4-88. In the nomenclature, the first number represents the viscosity in mPas of a 4% aqueous solution at 20 °C. This value is directly related to the molecular weight; thus, it is also an indicator for the viscosity of the melt. The second number stands for the percentage of hydrolyzed acetate groups and thus for the proportion of hydrophilic groups. Due to the difference in the hydrophilic/hydrophobic ratio, it is possible to partially interact with the API and prevent the API from precipitation [37].
In the literature, several solid and liquid plasticizers for PVA have already been described for different applications [38,39,40,41]. However, to the best of our knowledge, no research has comprehensively assessed the influence of plasticizers on the thermal, rheological, and mechanical properties of PVA in detail. To close this gap, we aimed to investigate and compare the two often-used PVA grades PVA 3-82 and PVA 4-88 regarding their key thermal parameters, their viscosity profiles in the melt, and the mechanical properties of the filaments produced via hot melt extrusion. Additionally, the influence of the plasticizers sorbitol (SOR), mannitol (MAN), glycerol (GLY), triacetin (TRI), triethyl citrate (TEC), and polyethylene glycol (PEG) in the grades PEG 300, PEG 400, PEG 600, and PEG 1000, and poloxamer (PLX) in grades 188 and 407 should be evaluated. The investigation of the plasticizers focuses in particular on the extension of the process window of the two PVA grades and furthermore, to compare the plasticizer influence on the mechanical properties of filaments. The different plasticizer combinations were ranked according to their influence on the rheological behavior of the melt and the mechanical properties of the PVA filaments. Finally, a statistical analysis by principal component analysis (PCA) should identify possible correlations between the data obtained and thereby provide a basic understanding of the interactions between plasticizer candidates and PVA. This is expected to provide new insights into the formulation development of PVA using HME.
Download the full article as PDF here: Understanding the Interaction of Thermal, Rheological, and Mechanical Parameters Critical for the Processability of Polyvinyl Alcohol-Based Systems during Hot Melt Extrusion
or read it here
Materials
Polyvinyl alcohol 3-82 (Parteck® MXP 3-82) and Polyvinyl alcohol 4-88 (Parteck® MXP 4-88) were supplied by Merck KGaA (Darmstadt, Germany).
With the exception of poloxamer 407 (Kolliphor®P 407, BASF SE, Ludwigshafen, Germany), all other plasticizers used (poloxamer 188 (Parteck® PLX 188), sorbitol (Parteck® SI 150), mannitol (Parteck® M200), PEG 300, PEG 400, PEG 600, PEG 1000, glycerol, triacetin, triethyl citrate) were supplied by Merck KGaA (Darmstadt, Germany).
Hess, F.; Kipping, T.; Weitschies, W.; Krause, J. Understanding the Interaction of Thermal, Rheological, and Mechanical Parameters Critical for the Processability of Polyvinyl Alcohol-Based Systems during Hot Melt Extrusion. Pharmaceutics 2024, 16, 472. https://doi.org/10.3390/pharmaceutics16040472
Are you interested in our webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


