How to improve API Bioavailability?

Today, poor solubility of New Chemical Entities is one of the most frequent and greatest challenges for formulators in the development of oral solid dosage forms. Improving API solubilization is thus of interest to increase bioavailability.
Have a look at SEPPIC’s newest solutions for bioavailability enhancers for poorly-soluble drug release (click to enlarge):
Some Advantages of SEPITRAP™
Use in direct compression
- Easy to use: simple mixing with other powders
- Gain time & cost: it cuts out process steps in comparison with wet granulation
- Avoid granulation for moisture sensitive APIs
- Avoid the degradation of the API without impacting the tablet properties
Dosage forms
- Tablets by direct compression
- Capsules
- Dry syrups
- Dry suspensions
Approved with various API
Arthemether + Lumefantrine, Nebivolol, Atorvastatin, Rivaroxaban, Ledipasvir, Albendazole, Simvastatin, Loratidine, Glimepiride* ( *Non exhaustive list. Request sales team for list of countries approvals.)
What is SEPITRAP™?
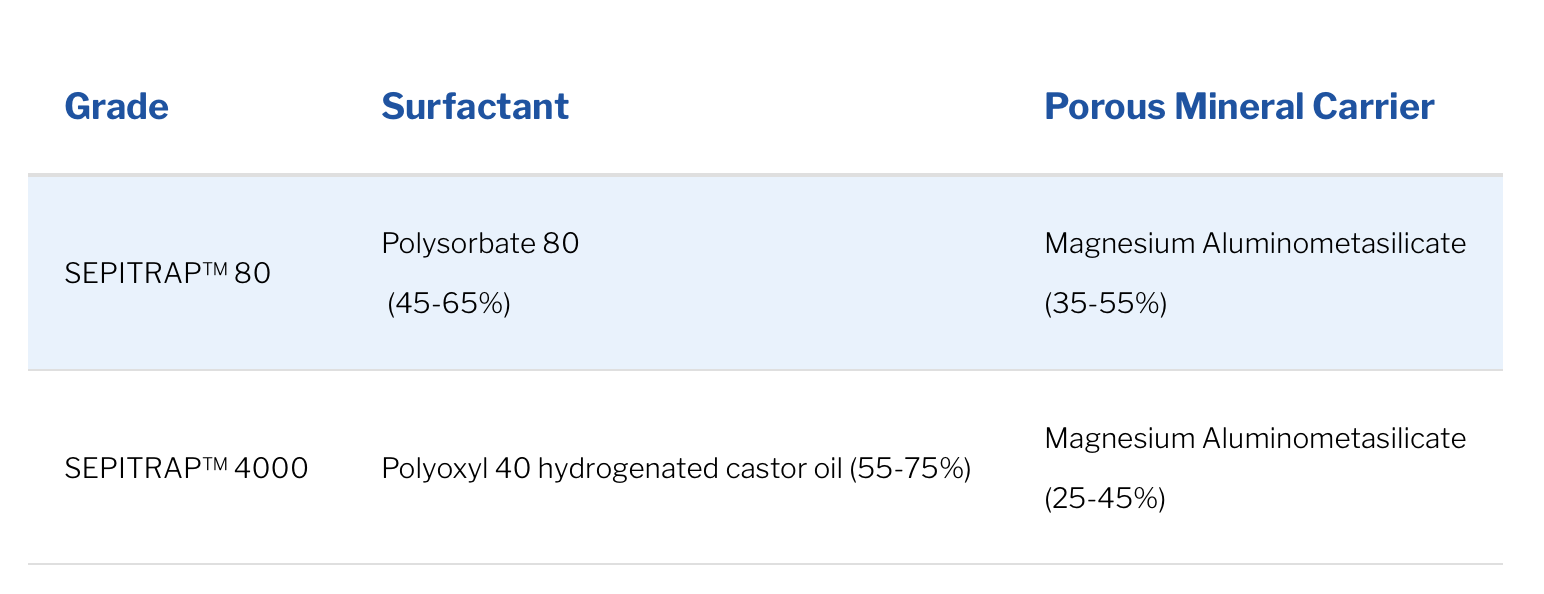
SEPITRAP™ is a micro-encapsulated solubilizer in powder form designed to simplify the manufacturing of solid oral form drugs. It is manufactured by adsorption of the solubilizer in liquid form on a porous support. SEPITRAP™ consists of a surfactant and a porous mineral carrier
SEPITRAP™ is both a solid solubilizer and a compression agent. It is a functional excipient designed specifically to simplify manufacturing of dry-form drugs while improving bioavailability of the active ingredient.
There are two solubilizers in the SEPITRAP™ range:
All the components of SEPITRAP™ comply with USP-NF. SEPITRAP™ is manufactured in accordance with IPEC GMP standards and is certified EXCiPACT.