Development of Imeglimin Electrospun Nanofibers as a Potential Buccal Antidiabetic Therapeutic Approach
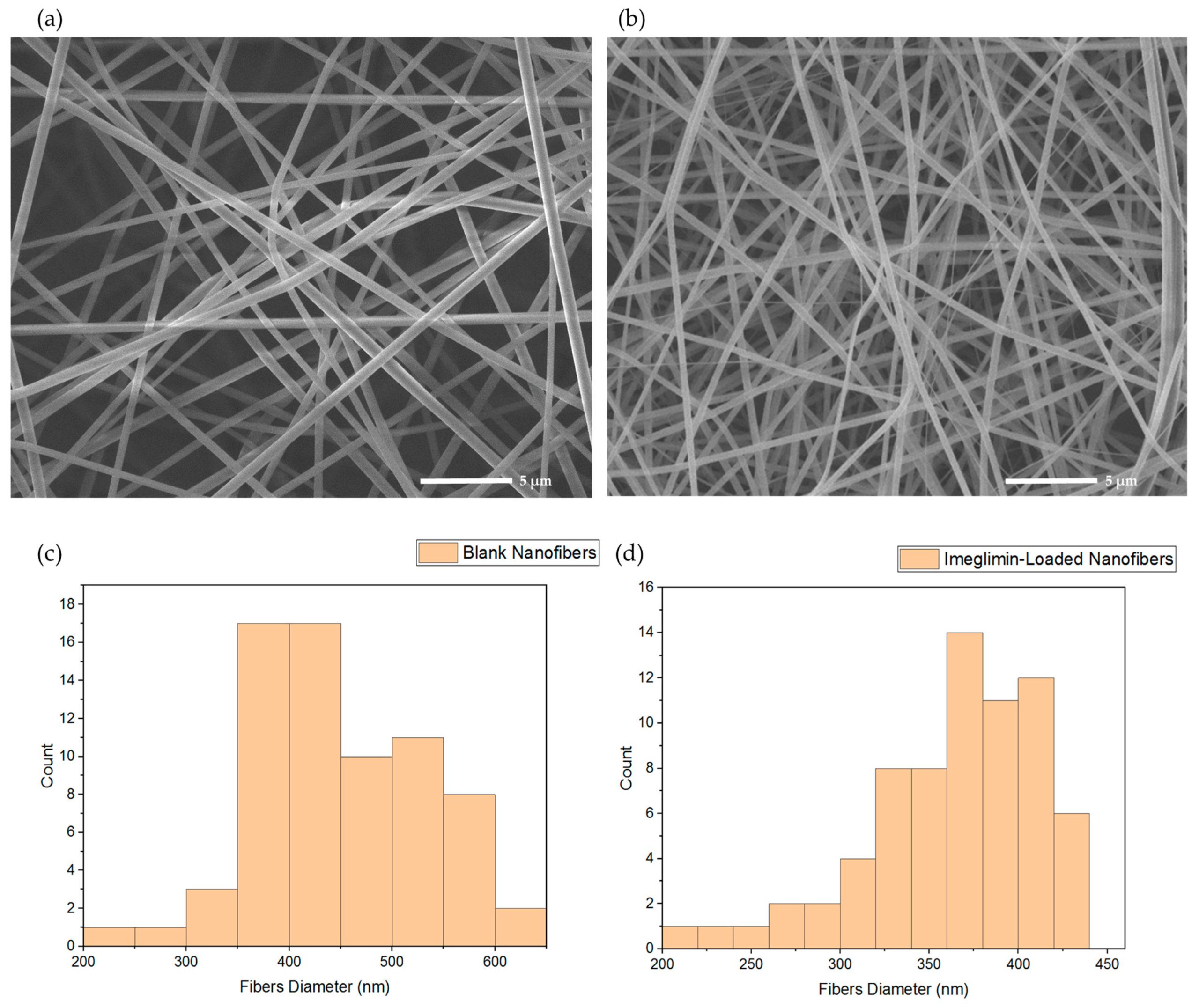
The prevalence of type 2 diabetes (T2D) has been growing worldwide; hence, safe and effective antidiabetics are critically warranted. Recently, imeglimin, a novel tetrahydrotriazene compound, has been approved for use in T2D patients in Japan. It has shown promising glucose-lowering properties by improving pancreatic beta-cell function and peripheral insulin sensitivity. Nevertheless, it has several drawbacks, including suboptimal oral absorption and gastrointestinal (GI) discomfort. Therefore, this study aimed to fabricate a novel formulation of imeglimin loaded into electrospun nanofibers to be delivered through the buccal cavity to overcome the current GI-related adverse events and to provide a convenient route of administration. The fabricated nanofibers were characterized for diameter, drug-loading (DL), disintegration, and drug release profiles. The data demonstrated that the imeglimin nanofibers had a diameter of 361 ± 54 nm and DL of 23.5 ± 0.2 μg/mg of fibers. The X-ray diffraction (XRD) data confirmed the solid dispersion of imeglimin, favoring drug solubility, and release with improved bioavailability. The rate of drug-loaded nanofibers disintegration was recorded at 2 ± 1 s, indicating the rapid disintegration ability of this dosage form and its suitability for buccal delivery, with a complete drug release after 30 min. The findings of this study suggest that the developed imeglimin nanofibers have the potential to be given via the buccal route, thereby achieving optimal therapeutic outcomes and improving patient compliance.
1. Introduction
Download the full study as PDF here: Development of Imeglimin Electrospun Nanofibers as a Potential Buccal Antidiabetic Therapeutic Approach
or continue reading here
Alamer, A.A.; Alsaleh, N.B.; Aodah, A.H.; Alshehri, A.A.; Almughem, F.A.; Alqahtani, S.H.; Alfassam, H.A.; Tawfik, E.A. Development of Imeglimin Electrospun Nanofibers as a Potential Buccal Antidiabetic Therapeutic Approach. Pharmaceutics 2023, 15, 1208.
https://doi.org/10.3390/pharmaceutics15041208

