Use of Excipients in Downstream Processing to Improve Protein Purification
Excipients are used to improve the stability of protein-based therapeutics by protecting the protein against a range of stress conditions such as temperature changes, pH changes, or agitation. Similar stresses are applied to proteins during downstream purification. Shifts in pH during Protein A chromatography, subsequent incubations at low pH for virus inactivation, and changes in conductivity in ion exchange chromatography can lead to aggregation, fragmentation, or other chemical modifications of the therapeutic protein. Given the potential impact on the protein’s structural integrity, there is a need for approaches to reduce the risk presented by the conditions during downstream processing. For example, integration of a solution to prevent aggregation of proteins would be a more efficient strategy than implementing steps to remove multimeric forms.
This white paper highlights the results from a recent paper by Stange et. al., in which protein stabilizing excipients such as polyols, sugars, and polyethylene glycol (PEG4000) were used as buffer system additives. Effect of the excipients on elution patterns, stabilization of the monomer antibody, host-cell protein removal, virus inactivation rates and binding capacity of cation exchange chromatography were explored. Results of the study show that addition of excipients can have beneficial effects on Protein A chromatography and virus inactivation, without harming subsequent chromatographic steps.1
Excipient Selection
The impact of excipients on chromatography performance has been evaluated in several studies.2,3,4 While the addition of excipients in Protein A chromatography buffers can improve column performance, a limited number of excipients and resins have been studied and the impact on subsequent steps was not explored.
The excipients for the study summarized below were selected using a specifically designed screening assay. Polyols, such as sorbitol and mannitol, are typically used as lyoprotectants,5,6,7 while osmolytes, such as trehalose and sucrose, are well-known stabilizers for protein formulations.7,8,9 PEG4000 is frequently used either as a partitioning agent in aqueous two-phase systems,10 as a precipitating agent,11 or to form covalent adducts (a process known as PEGylation) with proteins to enhance their size and prolong their biological half-life.12 A concentration of 500 mM or 5% (w/v) in the case of PEG4000 was used for the five excipients based on ideal stabilizing properties at the chosen concentration during the prescreening experiments.
Excipient Impact on Protein A Chromatography
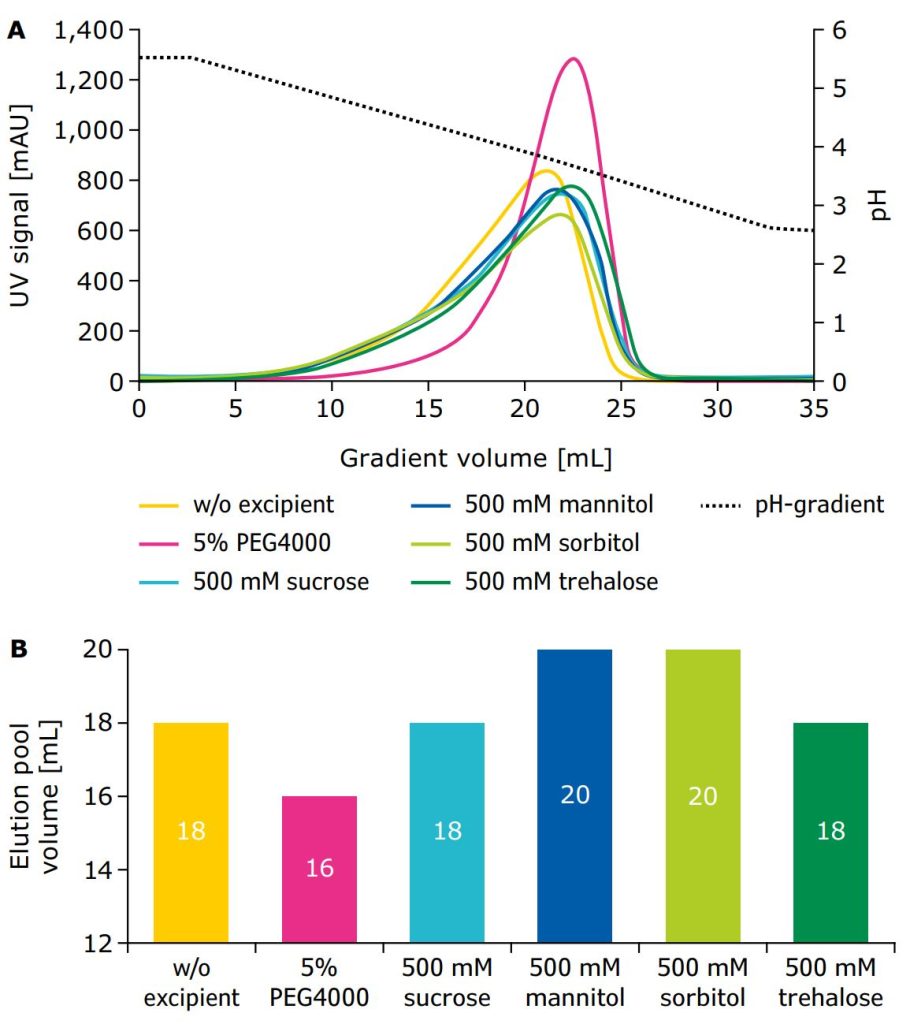
To determine the impact of different excipients on elution behavior in Protein A chromatography, excipients were introduced into the columns during the second wash step after loading and during elution of the monoclonal antibody (mAb). Normally, Protein A chromatography is performed with a step elution to a low pH; in this study, a linear gradient to low pH was used to more effectively evaluate the impact of excipients. Figure 1A shows representative results of the elution behavior on the Protein A column (Eshmuno® A column) using clarified cell culture harvest mAbB as model protein in the presence of various excipients compared to the control condition without an excipient. The addition of all selected sugars and polyols resulted in a fronting peak similar to the control condition without excipients. In comparison, the addition of PEG4000 led to a sharper and more symmetric elution peak slightly shifted to lower pH and resulted in a comparable or lower elution pool volume (Figure 1B). This was probably caused by interactions of PEG4000 with the bound antibody/Protein A ligand complex, which reduced peak fronting, and led to more symmetrical peak shapes of the eluting antibody. Previous studies showed that the antibody undergoes conformational changes during binding and elution of Protein A chromatography,13 that the hydration layer of the protein can be penetrated with PEGs through increasing concentration,14 and that PEGs can interact with nonpolar regions of a protein when exposed during conformational transitions.15 Therefore, PEG4000 could interact with and stabilize the conformationally altered form of mAbB/Protein A ligand complex during elution, which led to the observed shift in elution pH and narrowing and sharpening of the eluting antibody UV signal peak.
Because Protein A chromatography is commonly used to remove host cell proteins (HCPs) from cell culture harvests, the effect of excipients on their clearance was evaluated. For this purpose, fractions from all excipient conditions were collected during gradient elution and HCP concentrations were determined. The purity of the elution pool relative to HCP content was analyzed by comparison of HCP in the elution pool from collected fractions with total mAb content during the pH gradient.
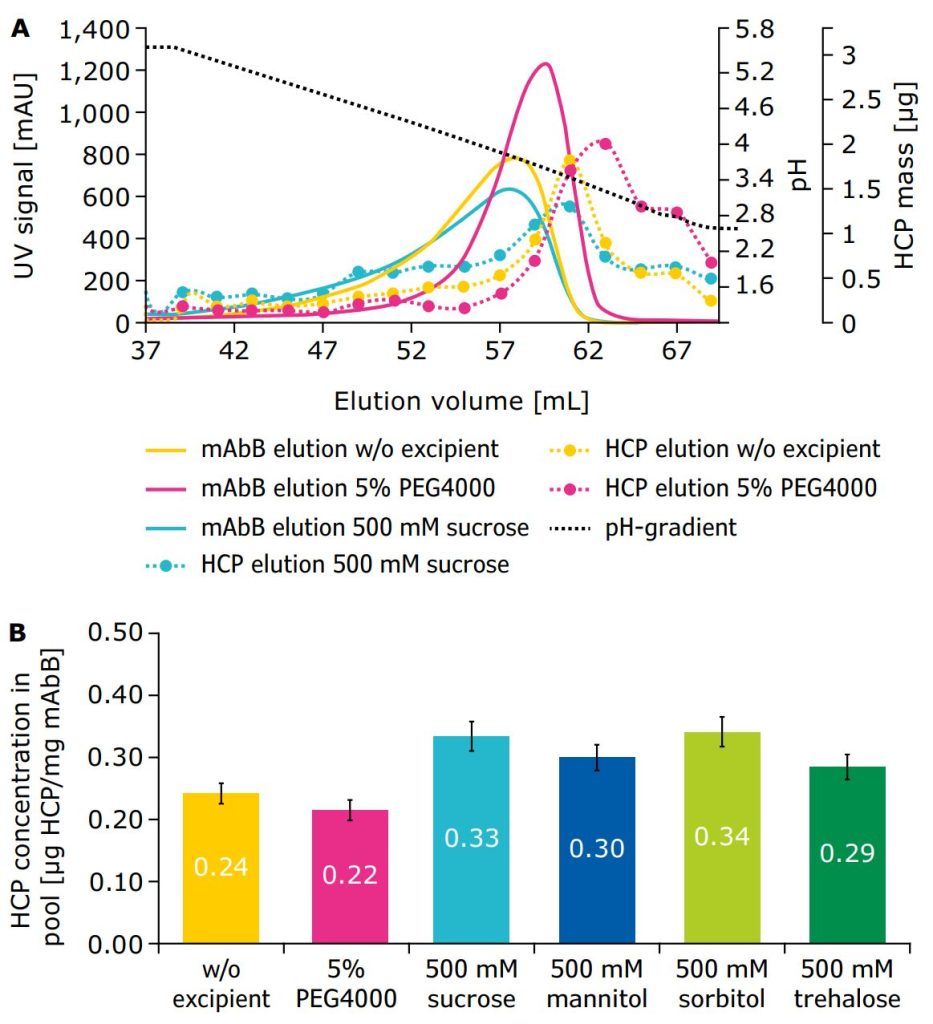
The elution behavior of HCPs in the presence of PEG4000 differed significantly from control and other excipient conditions (Figure 2A). With PEG4000, more HCPs were eluted in the rear part of the mAb elution peak, meaning the HCP elution was shifted to lower pH conditions and after the antibody desorbed from the column. HCP concentrations of elution pools were then quantified through ELISA for all excipients conditions. The greatest reduction of HCP level in the product pool, down to 0.22 μg HCP/mg mAbB, was achieved with the addition of 5% PEG4000 (Figure 2B).
Excipient Impact on Low pH Virus Inactivation
The potential stabilizing effects of excipients during the low pH hold used for virus inactivation were also investigated. While virus inactivation is typically performed at pH ≤3.8, in this case, the model antibodies were too stable, and no differences in aggregate level at pH values above 2.8 were observed. For this reason, the pH was adjusted to 2.8 after elution of the antibody from Protein A column to induce stress conditions in order to observe the stabilization effect of the excipients. The aggregate content was measured with size exclusion-HPLC directly after the elution pool was collected and then every 20 minutes over the course of one hour, after the pH was adjusted to 2.8. Under low pH conditions, the monomer content of mAbB decreased to approximately 93% in the control sample without excipient, while addition of sugars or polyols led to stable monomer content of about 99% within 60 minutes incubation at pH 2.8 (Figure 3). These results demonstrate that these excipients stabilized the protein at low pH condition. In contrast, PEG4000 showed no significant impact on mAb stability under this condition, as indicated by the monomer amount which was similar to the control without excipient.
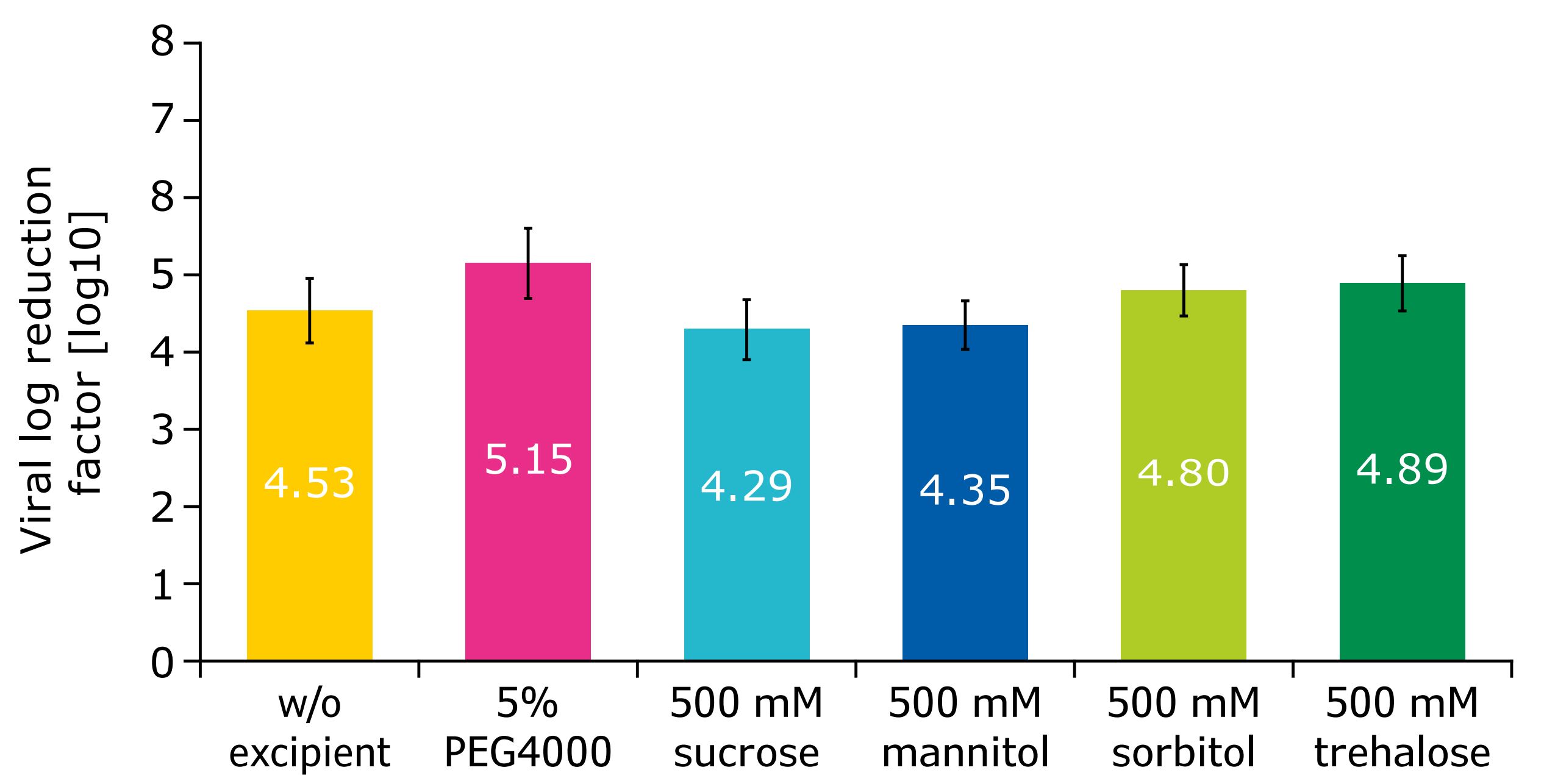
The effect of the selected excipients on viral inactivation rates was also studied. Viral log reduction factors were studied by using xenotropic murine leukemia virus (MLV) spiked into a solution of mAbB; virus inactivation was measured over several time points, up to one hour at pH 3.6. Similar to control experiments without excipients, all selected excipients showed highly effective virus reduction with a log reduction factor >4. Moreover, a slight improvement of the log reduction factors could be achieved by addition of PEG4000, sorbitol, and trehalose in comparison to the result without excipients (Figure 4). This result also demonstrated that the various excipients did not have a negative effect on virus inactivation.
Until this see the full interactive White Paper on “Use of Excipients in Downstream Processing to Improve Protein Purification” here
(click the picture to download the White Paper)
Source: Merck White Paper “Use of Excipients in Downstream Processing to Improve Protein Purification”
Do you need more information or a sample of excipients by Merck?


