Breaking barriers: Intranasal delivery of brexpiprazole-nanostructured lipid carriers targets the brain for effective schizophrenia treatment
With a peak occurrence in mid-teens to early adulthood, schizophrenia is one of the most debilitating illnesses, distinguished by hallucinations, dementia, psychosis, and delirium. According to figures from the World Health Organization, there are 21 million people who have schizophrenia worldwide, and India reports >1 million instances of psychosis each year [1]. Positive, negative, and cognitively dysfunctional symptoms are present upon beginning, typically occurring between the ages of 20 and 35. A patient with schizophrenia suffers grave consequences in many areas of their lives. One of the top 10 diseases in the world, it often shortens the patient’s life expectancy by ten years. During the first 4–6 weeks of treatment, more than 34% of patients exhibit therapeutic adherence issues; within the following two years, that percentage rises to 74%, leading to a noticeably high relapse rate, risk, and length of hospitalization.
Because they cause improved cognition, fewer extrapyramidal symptoms, less restlessness, and diminished tardive dystonia than traditional antipsychotic medications, atypical antipsychotics are more commonly used to treat schizophrenia (SCZ) [2]. SCZ is specified by aberrant glutamate and dopaminergic neurotransmission, hypofrontality, and synaptic inadequacy. Functional alterations in various frontal cortex subregions may finally explain why communication between large-scale brain networks is impeded. There is evidence of a neurochemical anomaly that affects the function of dopamine and glutamatergic N-methyl-d-aspartate receptors [3]. A key component of SCZ treatment is an antipsychotic drug. Numerous psychosocial interventions, comprising cognitive behavior therapy for psychosis, family support, disease self-management training, supported employment, social skills instruction, and assertive community treatment, are also advantageous [4]. Antipsychotic medications, antidepressants, psychotherapy, social support, and other treatments are currently accessible. Conventional therapies typically take the oral route, which might result in dose-related side effects due to non-specific targeting and pre-systemic metabolism. To decrease systemic adverse effects and improve therapeutic efficacy, it is necessary to design a site-specific formulation for SCZ.
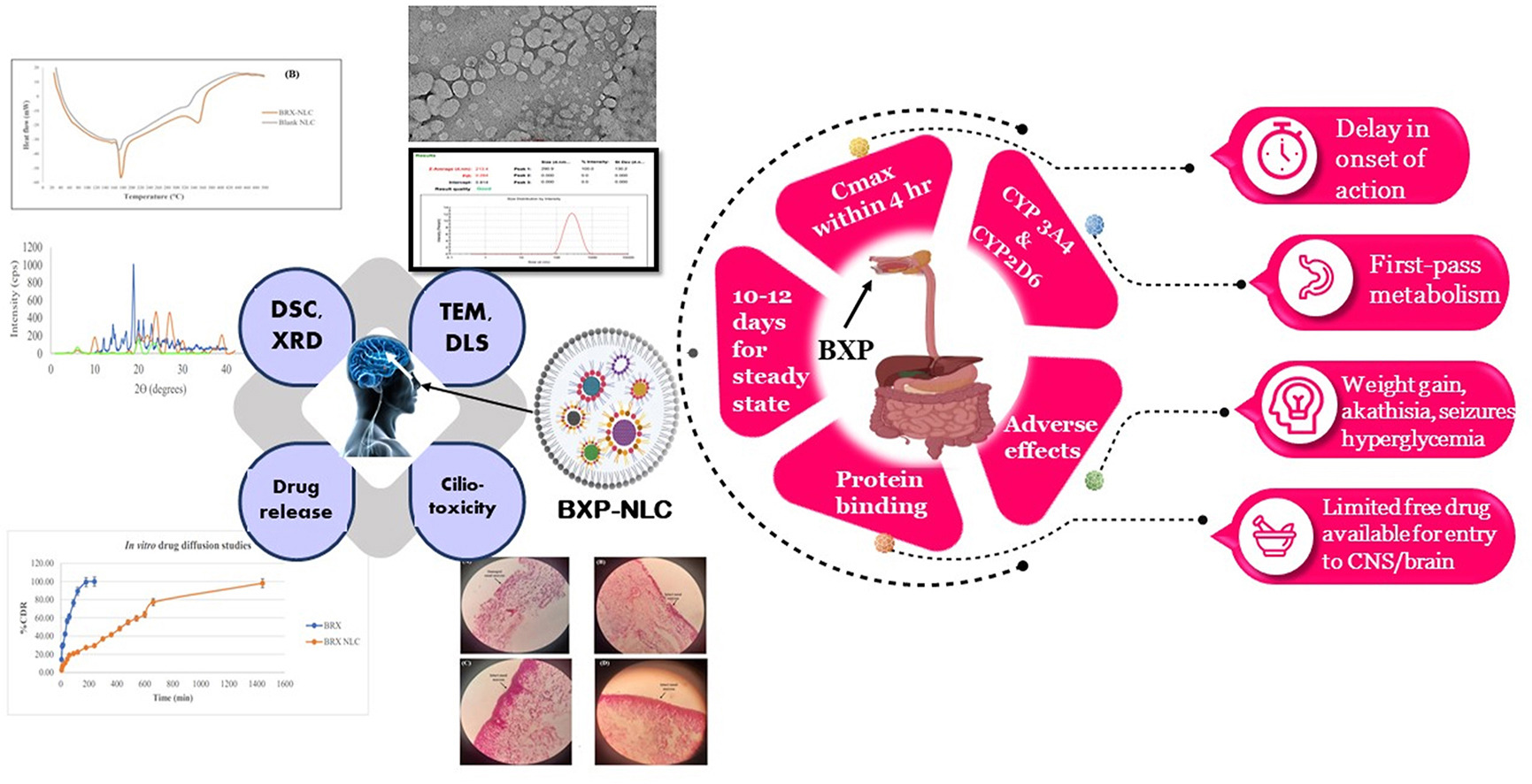
Brexpiprazole (BXP) is the medication of choice for treating SCZ. After 10–12 days of administration, steady-state concentrations of BXP are reached, and its oral bioavailability is 95%. However, few free drugs in circulation can enter the central nervous system (CNS) or brain because of considerable protein binding. It is a novel 5-HT2A receptor and noradrenergic 1B and 2C receptor partial agonist and potent antagonist of dopamine D2 and serotonin 1A (5-HT1A) receptors, respectively [5]. For treating SCZ and major depressive disorder, BXP was approved by the USFDA in July 2015 as a supplement to antidepressants for oral drug delivery [6]. The medicine has many dose-related side effects, including weight gain, akathisia, somnolence, tremor, seizures, hyperglycemia, etc. It is typically administered as a single or repeated daily dose to treat SCZ. The Blood Brain Barrier (BBB), which hampers the drug from reaching the CNS in sufficient quantities, is another problem. The BBB’s principal role is to obstruct the entrance of extraneous substances, comprising therapeutic drugs, which significantly impact drug delivery and distribution in the brain [7,8]. This means that the first option for treating SCZ is to cross the BBB, distribute the bio-actives across the BBB, or avoid the BBB altogether. A non-oral delivery system must be developed to treat SCZ and prevent/reduce adverse pharmacological effects.
The intranasal route stands out among other non-oral delivery methods for the brain since it is non-invasive and secure. Other non-oral delivery methods include parenteral, transdermal, vaginal, and rectal [9,10]. Owing to its porous endothelium membrane, high blood flow, vast surface area, and avoidance of pre-systemic metabolism, the nasal pathway has been reported to offer a new non-invasive drug delivery option for treating CNS illnesses [2,11]. Enhancing brain bioavailability could result in a lower dose of BXP, which would lessen any adverse side effects. Consequently, intranasal delivery of BXP to the CNS or brain is preferable and has various benefits. This method avoids the BBB and directs the drug’s delivery mechanism to the brain, shielding it from the gastrointestinal tract (GIT) and reducing or eliminating adverse effects. After nasal delivery, either olfactory or trigeminal neural pathways are opted by formulation, as they are close to the brain [12]. As a result, it avoids systemic drug distribution, increases bioavailability, and offers site-specific targeting.
Drug biodistribution may be altered by delivery systems based on nanotechnology. They can be designed to administer medications precisely [13]. Thus, nanotechnology has significantly impacted the creation of medication delivery systems to treat illnesses and disorders for which viable treatments have not been created [14,15]. Researchers have documented the use of various drug carriers for intranasal brain delivery. These include coated lipid nanoparticles (NPs), polymeric nanoparticles, liposomes, solid lipid nanoparticles (SLNs), nanostructure lipid carriers (NLCs), microemulsions, and nanosuspensions [16]. When both of them are compared, the lipid NPs perform better than the polymeric NPs due to the cytotoxicity of the polymeric NPs as well as the lack of efficient mass manufacturing methods. The first generation of lipid-based nanoparticles, SLNs, was initially characterized in the early 1990s [17]. Due to polymorphism, drug-carrying SLNs were limited by ineffective drug loading and a substantial risk of drug expulsion from the formulation during storage.
To address SLN formulation difficulties, NLCs were created as next-generation SLNs in the late 1990s [18]. By combining solid lipids (SL) and liquid lipids (LL), which provide an amorphous solid matrix at body and ambient temperatures, Müller et al. were able to make NLCs. The first and most crucial step was integrating LL into the matrix because LL markedly improved the formulation’s properties compared to SLNs [19]. LL assisted amorphous lattice development with significant holes in its solid crystalline matrix, allowing a higher drug load to be added, as opposed to SLNs, which form a solid crystalline matrix that restricts the amount of drug-loaded and causes its ejection due to their spatial capacity [20]. Numerous scientific research studies have shown that the NLC improves stability and loading effectiveness while limiting drug evacuation during storage, overcoming the limitations of the SLN [21,22]. NLC substantially boosts the solubility of lipophilic medications and makes drug absorption easier [23].
Additionally, it was determined in earlier investigations to be acceptable for the intranasal delivery of bioactive. It covers several nasal route drawbacks, such as enzymatic breakdown and inadequate nasal penetration. Through nasomucosal enzymes, it prevents the medication from being degraded and encourages nasal penetration. NLC does not irritate or harm the nasal mucosa because of its lower toxicity profile and naturally lipid-based nature [24].
When administered intranasally, a thermally reversible levetiracetam gel made by Goncalves et al. had a drug-targeting efficacy of 182% [25]. By delivering ketoconazole NLCs down the nose to the brain, Du et al. (2019) saw a considerable improvement in mice with cryptococcal meningoencephalitis [26]. Melatonin-loaded polycaprolactone nanoparticles were created and administered intranasally by De Oliveira et al. Fluorescence tomography pictures indicated a considerable advancement in drug targeting from the nose to the brain [27].
An intriguing method for delivering medicine straight to the brain via the nose is the “nose to brain” drug delivery. The olfactory region is the sole area of the body where the CNS interacts with the surrounding environment. Nasal mucosa facilitates faster and complete drug uptake straight to the brain, avoiding the BBB. This results in improved bioavailability, a lower dose, and fewer side effects [28]. Self-administration is possible through the intranasal route. NLCs are promising therapeutic delivery carriers for intranasal delivery. It offers advantages such as higher drug loading, better physical stability, scalability, biocompatibility, less toxicity, targeted delivery, etc [29].
This study sought to formulate and evaluate NLCs for enhancing the intranasal administration of BXP to the brain with the help of SL and LL, which ultimately helps to enhance the permeation of the drug through the nasal mucosa and improve the drug’s effectiveness [30]. The Design Expert (DoE, version 13, State-Ease Inc., Minneapolis, USA) software used Box-Behnken design (BBD) to optimize NLCs created via the melt emulsification sonication procedure. The prepared BRX-NLC was examined for physicochemical characterization. The optimized formulation was next tested for nasal ciliotoxicity, ex-vivo permeability, and in-vitro drug diffusion.
Read more here
Materials
BXP was a kind gift from Zydus Life Sciences Pvt. Ltd., Gujarat, India. Various lipids like GMS and Precirol A.T.O. 5 were provided as a gift sample from Gattefosse Pvt. Ltd., Mumbai (India). Compritol 888 Pellets, Maisine 35, Plurol oleique, CC 497, Peceol, Dynasan 114, Dynasan 116, Dynasan 118, Captex 300, Captex 355, Sefsol 218, Labrafil M 1944 CS, Tween 80, Cremophor EL, Cremophor RH 40 were gifted from Department of Pharmaceutics, Ramanbhai Patel College of Pharmacy (RPCP)
Shailvi Shah, Amit A. Patel, Vidhi Pandya, Nidhi Trivedi, Samir G. Patel, Bhupendra G. Prajapati, Sudarshan Singh, Ravish J. Patel, Breaking barriers: Intranasal delivery of brexpiprazole-nanostructured lipid carriers targets the brain for effective schizophrenia treatment, Journal of Drug Delivery Science and Technology, Volume 90, 2023, 105160, ISSN 1773-2247, https://doi.org/10.1016/j.jddst.2023.105160.

