Development of famotidine-loaded lecithin-chitosan nanoparticles for prolonged and efficient anti-gastric ulcer activity
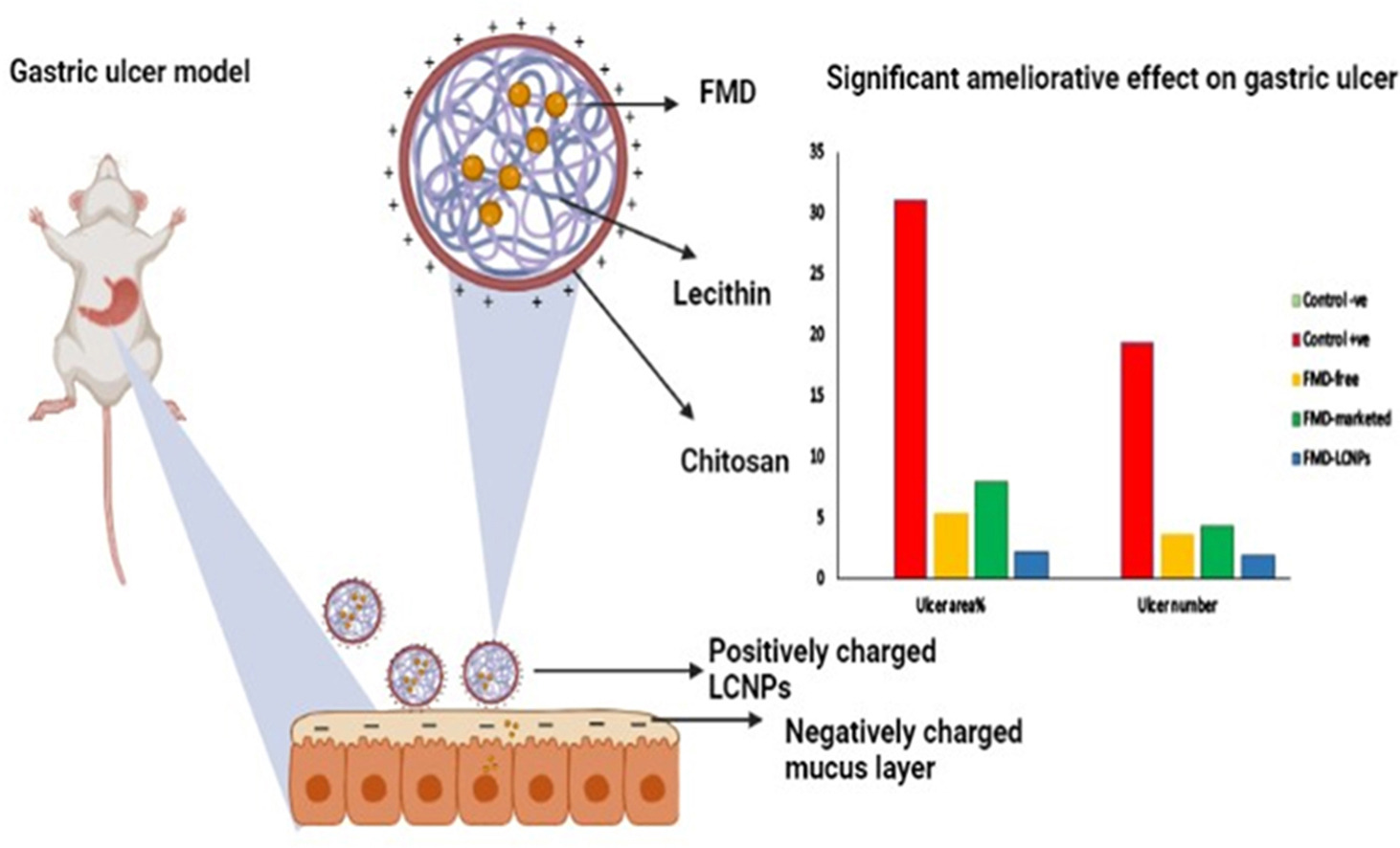
Lecithin-chitosan nanoparticles (LCNPs) represent an appealing nanocarrier for the oral delivery of hydrophobic drugs. Negatively charged lecithin and positively charged chitosan were permitted to interact to create LCNPs. Their distinctive features enable drugs to be encapsulated into the lipophilic lecithin core while improving their retention in gastric mucosa by the chitosan coat. Famotidine (FMD), a hydrophobic H2-receptor antagonist drug with a short half-life (2.5–4 h), was loaded into LCNPs to enhance its therapeutic efficacy in gastric ulcers through achieving good mucoadhesion and subsequent absorption. The optimal formula with a lecithin to chitosan ratio of 10:1 was selected and exhibited a particle size of 178.5 ± 9.5 nm, a zeta potential of 43.7 ± 2 mV, and an entrapment efficiency of 75.81 ± 2.5 %. It displayed a prolonged in vitro release profile (75.72 % over 24 h) and considerable mucoadhesive properties. FMD-LCNPs were also characterized by differential scanning calorimetry, Fourier transform-infrared (FTIR) spectroscopy, transmission electron microscopy, and stability studies. The in vivo study was performed on ethanol-induced gastric ulcer in a rat model, and the anti-gastric ulcer effects of FMD-free, FMD-marketed product, and FMD-LCNPs were determined by assessing the ulcer severity, antioxidant biomarkers, eNOS levels in addition to histopathological examination. FMD-LCNPs administration resulted in a percentage of protection from gastric severity of 96.25 % and an exceptional ameliorative effect on oxidative stress biomarkers (GSH; 162.36 % increase and MDA; 71.91 % decrease) as compared to other FMD forms. Additionally, FMD-LCNPs showed the strongest eNOS level improvement (130.34 %), which indicated the best healing impact and was confirmed by restoring the gastric mucosa to normal architecture.
Introduction
Gastric ulcer is a common and disturbing digestive system disorder affecting many people worldwide. It occurs when the mucosal protective factors, such as mucin, prostaglandins (PGs), antioxidants, bicarbonate, and nitric oxide, are outweighed by mucosal aggressive factors (e.g., Helicobacter pylori infection, continuous use of non-steroidal anti-inflammatory drugs (NSAIDs) and hypersecretion of pepsin and hydrochloric acid). This imbalance leads to a compromised mucosal protective barrier, forming gastric ulcer [1,2]. A variety of pharmacological classes are available for the treatment of gastric ulcers, including proton pump inhibitors, histamine H2-receptor antagonists, anticholinergic drugs, and antacids [1,3].
Among H2-receptor antagonists, famotidine (FMD) represents a more attractive option for gastric ulcer treatment, being more potent than ranitidine (7.5 folds) and cimetidine (20 folds) [4]. However, the therapeutic efficacy of FMD is restricted by many factors, including limited oral bioavailability (40–45 %) due to low solubility and permeability (BCS class IV) [5,6] and short half-life (2.5–4 h) [5,7]. On the other hand, nanotechnology approaches have a remarkable impact on improving oral drug delivery for gastric ulcers and various diseases and conditions. This is attributed to the potential of nanocarriers to regulate their physicochemical features by modifying their surface characteristics, morphology, and size. Preventing GIT destabilization of active ingredients, enhancement of drug permeability through the gut wall, improving aqueous solubility of hydrophobic drugs, and achieving drug targeting can be accomplished upon employment of nano-based systems [8,9]. Different nanocarriers were reported to enhance FMD efficacy through enhancement of its bioavailability and/or prolonging its action, such as solid lipid nanoparticles [10], self nano-emulsifying drug delivery system (SNEDDS) [11], niosomes [5], mesoporous silica nanoparticles [12] and graphene oxide nanoparticles [13].
Chitosan is a natural polysaccharide formed through chitin deacetylation. It has brought much attention due to its safety, biocompatibility, biodegradability, and applicability in various aspects, including oral drug delivery. Chitosan’s solubility is greatly influenced by pH; it is soluble in acidic conditions only [14]. Besides its several biological impacts, including antibacterial, antioxidant, anti-inflammatory, anti-diabetic, and anticancer activities [15,16], chitosan was reported to have an anti-ulcerogenic effect [17]. Including chitosan in nanocarriers can increase their mucoadhesive features, enhance their stability in GIT fluids, and improve drug absorption due to the ability of chitosan to open tight junctions within epithelial cells [14,18]. Furthermore, the mucoadhesive properties of chitosan prolong the duration of nanosystems in the gastrointestinal tract (GIT) [19], which is beneficial for treating stomach ulcers [20,21].
Lecithin, a negatively charged natural phospholipid with considerable safety and high demand in fabricating various nanocarriers, can interact with polycationic chitosan-forming lecithin-chitosan nanoparticles (LCNPs) [22]. Lipophilic drugs can be enclosed in the hydrophobic lecithin that makes up the inner core, while the hydrophilic chitosan composes the outside shell and stabilizes the particles [23]. LCNPs provide several advantages, such as extending drug residence time, achieving controlled release patterns, enhancing stability, and improving bioavailability [24,25]. Improvement of oral delivery of various hydrophobic drugs was accomplished using LCNPs for Artesunate [26], hydrochlorothiazide [27], curcumin [24], and raloxifene [19].
It is expected that targeting mucin by ionic interaction with formulations comprising positively charged groups will increase the formulation’s residence time, prolonging drug action and promoting the bioavailability and efficacy of the incorporated drug [28]. Previous literature demonstrated the advantageous features of mucoadhesive systems loaded with FMD. Using the media milling technique, Patel et al. developed FMD-based mucoadhesive nanosuspension comprising zirconium oxide beads, PVPK-30, and Tween-80. Results from in vivo investigations on aspirin-induced rats showed that FMD-based mucoadhesive nanoparticles reduced the ulcer index more significantly than conventional FMD suspension [29]. Thiolated polyacrylic acid microspheres loaded with both FMD and clarithromycin were developed by Srivastava et al. as a mucoadhesive delivery system for potentially treating peptic ulcers [30].
Furthermore, mucoadhesive chitosan-coated niosomes of FMD were developed by Khalifa et al. and exhibited good permeability, stability in bile salts, prolonged release, and elevated mucoadhesive efficiency [5]. Herein, LCNPs, with their mucoadhesive features and considerable ability to encapsulate lipophilic drugs, are suggested to be an appealing delivery system for FMD. So, the study aims to prepare lecithin-chitosan nanoparticles (LCNPs) loaded with famotidine (FMD) for the first time to prolong the drug action, enhance its effectiveness, and achieve better amelioration of gastric ulcers.
Read more
Moataz B. Zewail, Sanaa A. El-Gizawy, Gihan F. Asaad, Walaa A. El-Dakroury, Development of famotidine-loaded lecithin-chitosan nanoparticles for prolonged and efficient anti-gastric ulcer activity, Journal of Drug Delivery Science and Technology, Volume 91, 2024, 105196, ISSN 1773-2247,
https://doi.org/10.1016/j.jddst.2023.105196.


