Lipid-based particle engineering via spray-drying for targeted delivery of antibiotics to the lung
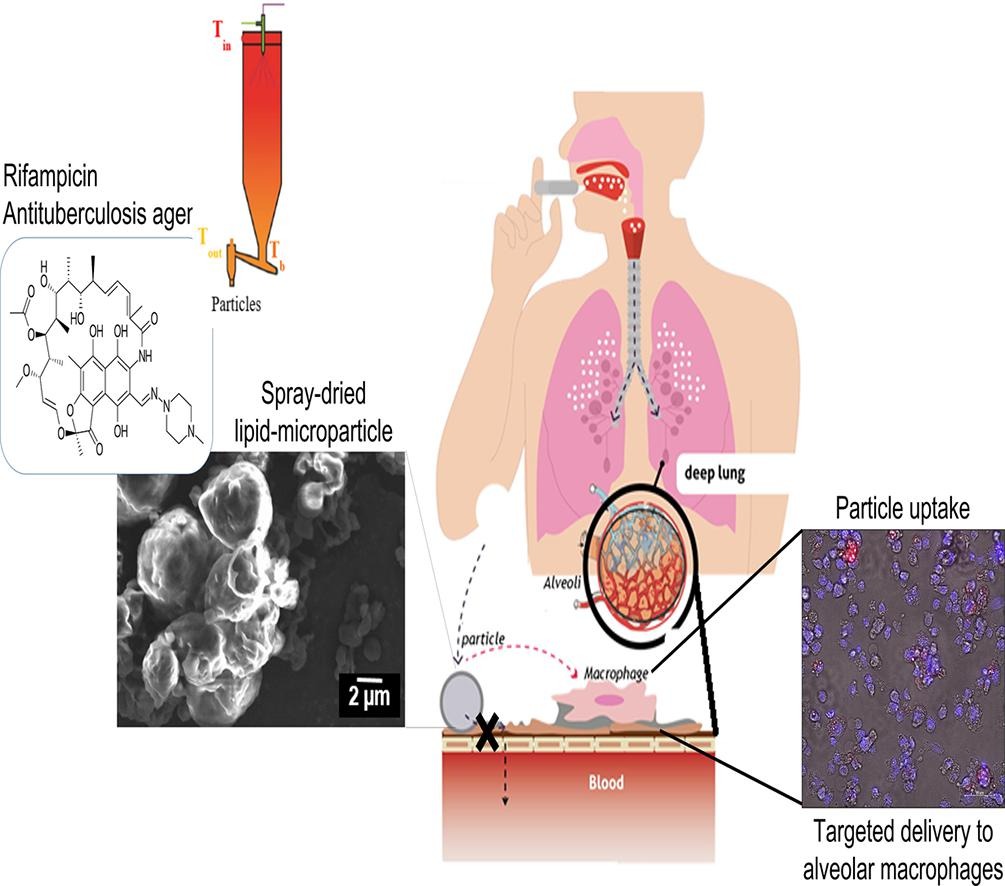
Pulmonary delivery of antibiotics for the treatment of tuberculosis provides several benefits compared to conventional oral and parenteral administration. API-loaded particles delivered directly to alveolar macrophages, where Mycobacterium tuberculosis resides, can reduce the required dose and decrease the severe side effects of conventional treatment. In this work, lipid-microparticles loaded with rifampicin were engineered via spray-drying to be administered as a carrier-free dry powder for inhalation. Although, it is well-known that spray-drying of lipid-based excipients is strongly limited, a completely lipid-based formulation using diglycerol full ester of behenic acid was produced. The solid state of the lipid, providing high melting temperature, absence of polymorphism and monophasic crystallization, led to high yield of spray-dried particles (83%).
Inhalable particles of mass median aerodynamic diameter of 2.36 µm, median geometric size of 2.05 µm, and negative surface (-50.03 mV) were engineered. Such attributes were defined for deep lung deposition and targeted delivery of antibiotics to alveolar macrophages. Superior aerodynamic performance as carrier-free DPI was associated to a high fine particle fraction of 79.5 %. No in vitro cytotoxic effects were found after exposing epithelial cell lines and alveolar macrophages. In vitro uptake of particles into alveolar macrophages indicated the efficiency of their targeted delivery. The use of highly processable and safe lipid-based excipients for particle engineering via spray-drying can extend the availability of materials for functionalized applications for pulmonary delivery.
Read more here
Materials
Diglycerol full ester of behenic acid, PG2-C22 full (Tm = 72.5 °C, HLB = 1.8), a molecule belonging to the PGFA group was selected as processable LBE for spray-drying. As shown in Fig. 1a, its chemical structure consists of two glycerol moieties bonded through an ether linkage and fully esterified with behenic acid. PG2-C22 full is synthesized by IOI Oleo GmbH (Witten, Germany) with the commercial name of Witepsol® PMF222.
Carolina Corzo, Djana Crvenjak, Kamen Sotirov, Jesus Afonso Urich, Kristin Öhlinger, Claudia Meindl, Dirk Lochmann, Sebastian Reyer, Eleonore Fröhlich, Andreas Zimmer, Sharareh Salar-Behzadi, Lipid-based particle engineering via spray-drying for targeted delivery of antibiotics to the lung, International Journal of Pharmaceutics, Volume 642, 2023, 123201, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123201.
Watch our free webinar “Spray Drying” here:


