Long-acting microneedle formulations
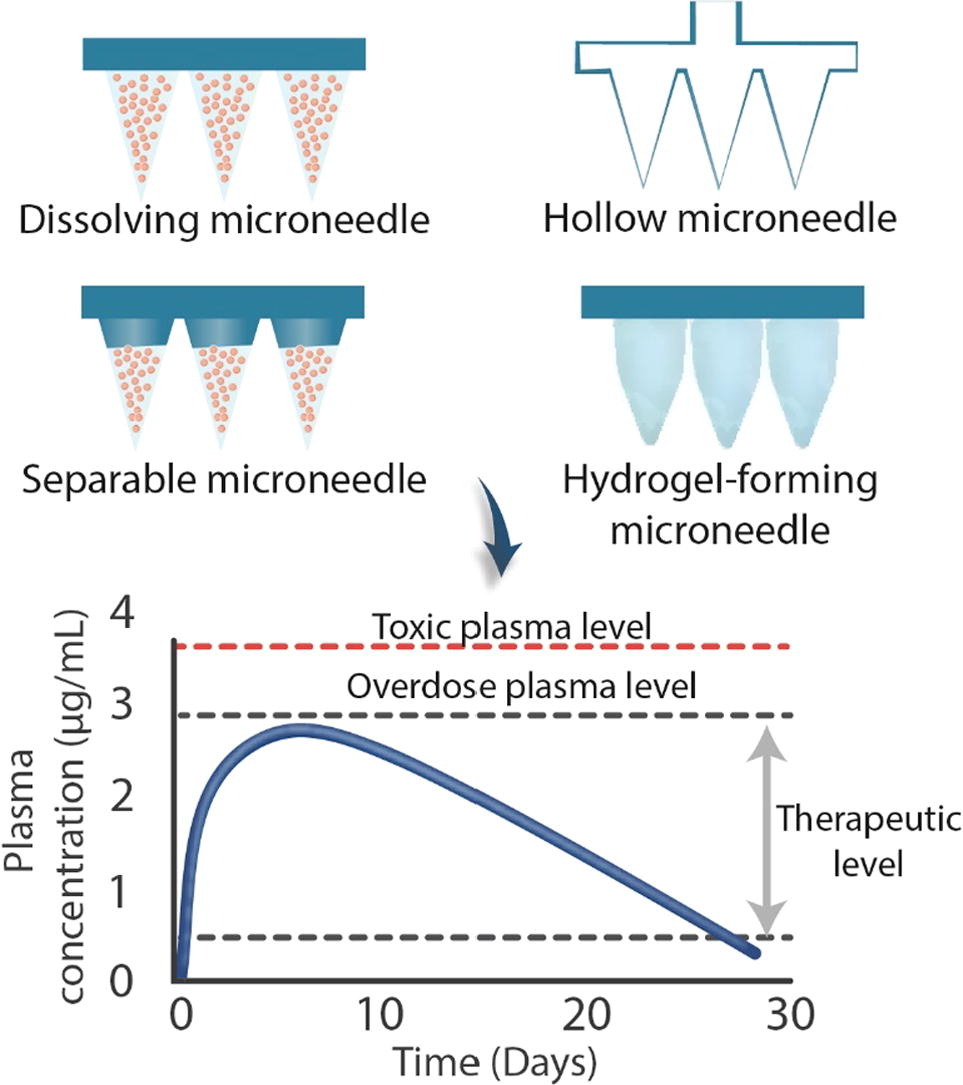
The minimally-invasive and painless nature of microneedle (MN) application has enabled the technology to obviate many issues with injectable drug delivery. MNs not only administer therapeutics directly into the dermal and ocular space, but they can also control the release profile of the active compound over a desired period. To enable prolonged delivery of payloads, various MN types have been proposed and evaluated, including dissolving MNs, polymeric MNs loaded or coated with nanoparticles, fast-separable MNs hollow MNs, and hydrogel MNs. These intricate yet intelligent delivery platforms provide an attractive approach to decrease side effects and administration frequency, thus offer the potential to increase patient compliance. In this review, MN formulations that are loaded with various therapeutics for long-acting delivery to address the clinical needs of a myriad of diseases are discussed. We also highlight the design aspects, such as polymer selection and MN geometry, in addition to computational and mathematical modeling of MNs that are necessary to help streamline and develop MNs with high translational value and clinical impact. Finally, up-scale manufacturing and regulatory hurdles along with potential avenues that require further research to bring MN technology to the market are carefully considered. It is hoped that this review will provide insight to formulators and clinicians that the judicious selection of materials in tandem with refined design may offer an elegant approach to achieve sustained delivery of payloads through the simple and painless application of a MN patch.
Introduction
The treatment of chronic diseases poses significant negative effects to patients’ day-to-day activities and has significant associated healthcare costs. Moreover, current treatment options for chronic diseases using conventional immediate-release delivery systems are associated with poor long-term patient compliance, incorrect dosing due to dose missing or skipping. Moreover, multiple administrations provide a “peak and valley” effect for drug plasma levels. The “peak” drug levels can lead to undesired side effects, while “valley” levels could be subtherapeutic, leading to treatment failure [1]. Long-acting drug delivery systems circumvent many of these issues associated with current treatment options for chronic diseases and have the advantage of controlled drug delivery for improved therapeutic effects, decreased dosing frequency and avoidance of potential toxicity[2], [3], [4]. Interest in long-acting drug delivery has increased substantially in the last decade [5], [6]. This type of system offers the possibility of providing drug release for a prolonged period with a single administration as opposed to multiple drug administration using conventional drug delivery systems [5]. Moreover, the oral drug route, which is the favoured route for patients, offers significant challenges such as first-pass metabolism, inter-individual pharmacokinetic variability and limited stability of the drug within the gastrointestinal tract. Lastly, it is notable that the oral route presents other challenges related to patient compliance when pharmacological treatment is required chronically or for prolonged periods of time [7]. Long-acting drug delivery methods are a promising technique to overcome these issues [1], [8], [9], [10], [11].
Currently, long-acting drug delivery systems are used for the management of chronic conditions such as schizophrenia [12], [13], [14], HIV [15], ocular disease [16], [17], [18], diabetes [19], hypothyroidism [20], [21] or endocrine conditions [22], [23]. This is particularly interesting consideration that due to the rise in life expectancy within the global population, the occurrence of chronic conditions is assumed to increase worldwide [24]. In addition to the treatment of chronic conditions, long-acting drug delivery modalities are used in the treatment of other disease modalities, such as cancer [25], [26], [27], [28], [29], [30] and infections [31], [32]. Again, this is another critical area of application considering that cancer and infectious illnesses are the leading causes of death worldwide. The majority of the drug delivery systems currently available commercially or described in the literature are based on drug-loaded implants or injectable formulations [5]. This approach typically requires an injection or minor surgery to be implanted into the body of the patient [33]. This process is innately painful and cumbersome for patients and may lead to minor bruising post-surgery [34]. In addition, the prevalence of patients with trypanophobia and needle phobia complicates the administration process even further [35]. Therefore, there is a strong impetus to develop minimally invasive methods to administer the long-acting drug delivery systems. Over the years, it has become increasingly apparent that the use of microneedles (MNs), as a fast rising healthcare technology, can be applied to solve this issue.
MNs are minimally invasive systems containing microprojections that efficiently pierce the outmost layer of the skin, the stratum corneum (SC). They typically consist of several microprojections assembled on a supporting base, with length ranging from 25 to 900 μm [36]. By breaching the SC, MNs are capable of enhancing the delivery of a wide variety of therapeutics ranging from small drug molecules [37], [38], [39], [40] to biomacromolecules [41], [42], [43], [44] or vaccines [45], [46], [47]. In this way, MNs allow transdermal administration of drugs that are not capable of diffusing passively through the skin using conventional transdermal formulations. Moreover, MNs can create pores in the skin without stimulating the dermal nociceptor or reaching blood vessels due to their short length (normally shorter than 1 mm) [38].
Although the patent for MN technology was first filed in 1976 by Gerstel and Place from Alza Corporation, it was not until the advent of microfabrication techniques, arising from the field of microelectronics in the 1990s, that studies on utilising MNs for drug delivery began to emerge. The inventions of microfabrication tools coupled by innovation with the field of drug delivery science has led to development of an assortment of MNs with varying dimensions, sizes and materials. Following the first publication on MNs by Henry et al in 1998, the number of research articles and patents pertaining have seen an exponential growth within the field [48]. Innovation within the field of pharmaceutical science coupled with the ever-growing demand to manage complex diseases soon led to the development of dissolving MNs in 2006 [49]. By judicious selection of the MN matrix coupled with critical considerations of MN design, scientists are able to fabricate MN systems which are capable of delivering their payload within a couple of minutes or provide sustained release for over several months [50], [51]. Despite being almost five decades since Place and Gerstel filed their first MN patent, most MN-based products that are available on the market are indicated for cosmetic purposes with none being indicated for clinical purpose. Nevertheless, based on the trajectory of the current MN research as well as the ever-growing innovation within the field, it is not unreasonable to assume that within the foreseeable future the first MN product for clinical indications will be approved.
MNs, as a transdermal delivery system, have an advantage over conventional oral formulations due to their ability to bypass first-pass metabolism [38]. In addition, these devices can be utilised for painless self-administration of injectable formulations, eliminating the need for healthcare professionals. This feature allows patients to apply the injections themselves, making them suitable for use in remote areas where refrigeration and storage may not be readily available. The optimal design of MN devices involves considering multiple parameters that impact drug delivery efficiency, mechanical strength, and manufacturability. MN patches are crucial in biomedical research and are well tolerated in clinical trials [52]. They leave behind only biocompatible, dissolvable materials, which are safer than the biohazardous waste produced by needles and syringes [53], [54]. These advantages make MN technology an outstanding method for the administration of long-acting formulations [55]. Over the last two decades, several studies have been caried out and published describing the use of MN arrays for the delivery of long-acting formulations [56], [57], [58], [59], [60], [61].
The overarching goal of this review is to present an informed summary of the use of MNs as a potential pharmaceutical platform for the delivery of payloads in a sustained and prolonged fashion, which is of great clinical importance. We shall first provide a brief overview on the type of MNs that are frequently utilized for long-acting drug delivery. Then, we discuss the design strategies that have been implemented and investigated to achieve a long-acting drug delivery profile. Afterwards, we highlight the utility of computer modeling in streamlining microneedle design in combination pharmacokinetic-pharmacodynamic (PK-PD) data to enable formulators to make a more judicious and critical decision in fabricating MNs that are capable of delivering the required therapeutic dose in a sustained fashion. Later, we converse the evolution of MN-assisted delivery in the field of long-acting formulations, highlighting the pioneering work that has contributed to its current application.
Read more
Lalitkumar K. Vora, Akmal H. Sabri, Yara Naser, Achmad Himawan, Aaron R.J. Hutton, Qonita Kurnia Anjani, Fabiana Volpe-Zanutto, Deepakkumar Mishra, Mingshan Li, Aoife M Rodgers, Alejandro J. Paredes, Eneko Larrañeta, Raghu Raj Singh Thakur, Ryan F. Donnelly, Long-acting microneedle formulations, Advanced Drug Delivery Reviews, 2023, 115055, ISSN 0169-409X,
https://doi.org/10.1016/j.addr.2023.115055.

