Formulation Development and Improved Stability of a Combination Measles and Rubella Live-Viral Vaccine Dried for Use in the NanopatchTM Microneedle Delivery System
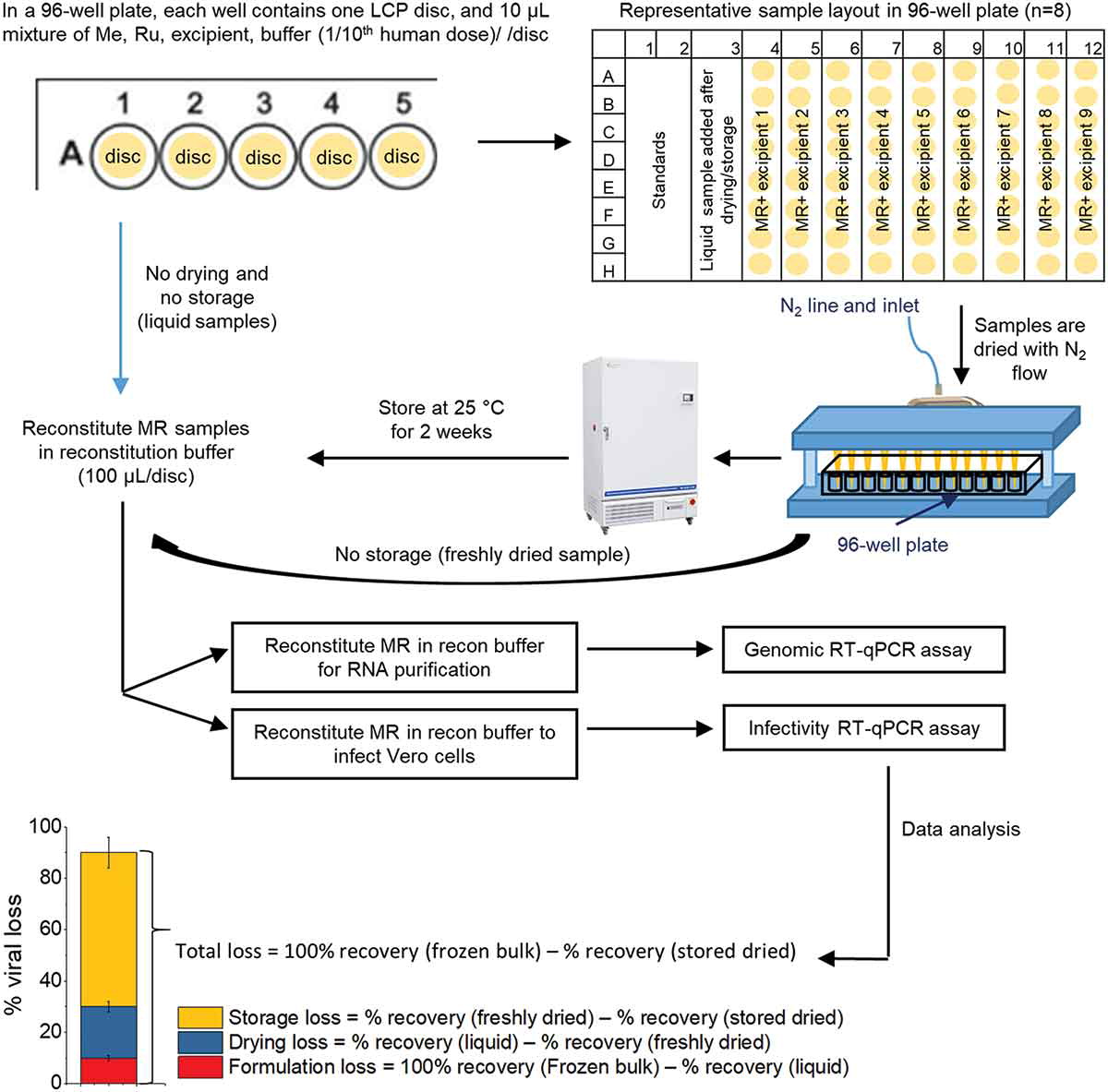
Measles (Me) and rubella (Ru) viral diseases are targeted for elimination by ensuring a high level of vaccination coverage worldwide. Less costly, more convenient MeRu vaccine delivery systems should improve global vaccine coverage, especially in low – and middle – income countries (LMICs). In this work, we examine formulating a live, attenuated Me and Ru combination viral vaccine with Nanopatch™, a solid polymer micro-projection array for intradermal delivery.
First, high throughput, qPCR-based viral infectivity and genome assays were established to enable formulation development to stabilize Me and Ru in a scaled-down, custom-built evaporative drying system to mimic the Nanopatch™ vaccine coating process. Second, excipient screening and optimization studies identified virus stabilizers for use during the drying process and upon storage in the dried state. Finally, a series of real-time and accelerated stability studies identified eight candidate formulations that met a target thermal stability criterion for live vaccines (<1 log10 loss after 1 week storage at 37°C).
Compared to −80°C control samples, the top candidate formulations resulted in minimal viral infectivity titer losses after storage at 2–8°C for 6 months (i.e., <0.1 log10 for Me, and ~0.4 log10 for Ru). After storage at 25°C over 6 months, ~0.3–0.5 and ~1.0–1.4 log10 titer losses were observed for Me and Ru, respectively, enabling the rank-ordering of the stability of candidate formulations. These results are discussed in the context of future formulation challenges for developing microneedle-based dosage forms containing stabilized live, attenuated viral vaccines for use in LMICs.
Download the full article as a PDF here or read it here
Materials: Measles bulk (Edmonston-Zagreb Strain, Clarified Virus Pool No. 066M626084), rubella bulk (Wistar 27/3 Strain, Clarified Virus Pool No. 068R508026), and anti-rubella serum (equine origin), were provided by Serum Institute of India Pvt. Ltd. (SIIPL), India, and were stored at −80°C (Frozen bulk). The concentration of each virus bulk was 105.32 CCID50/0.5 mL in a minimum essential medium (MEM), pH 7.2 with various excipients added. An in-house developed 96-well microplate drying rig was provided by Vaxxas Pty Ltd, Australia. Six millimeter diameter liquid crystal polymer (LCP) discs were provided by Cyrus Technology Pvt. Ltd., Singapore. Taqman™ Fast Virus 1-Step master mix and TaqPath™ 1-Step multiplex Master Mix TaqMan® were purchased from Applied Biosystems. Glutamic acid and lactose were obtained from Fluka. Human serum albumin (HSA) was purchased from Octapharma. Methionine was procured from MP biomedicals. Urea was purchased from Promega. Pluronic F-68 (PF68), dextran sulfate, and PEG-3350 were obtained from Spectrum Chemicals. Dithiothreitol (DTT), polysorbate 20 and 80 (PS20 and PS80), triton X-100 were obtained from ThermoFisher. Sucrose, trehalose, and mannitol were purchased from Pfanstiehl. Sulfobutyl ether beta-cyclodextrin (Captisol) was obtained from Ligand Technology. Sodium hyaluronate was obtained from Acros. All other excipients or reagents were purchased from Sigma-Aldrich.
Article information: (2021) Formulation Development and Improved Stability of a Combination Measles and Rubella Live-Viral Vaccine Dried for Use in the NanopatchTM Microneedle Delivery System, Human Vaccines & Immunotherapeutics, DOI: 10.1080/21645515.2021.1887692

