High loading of lipophilic compounds in mesoporous silica for improved solubility and dissolution performance
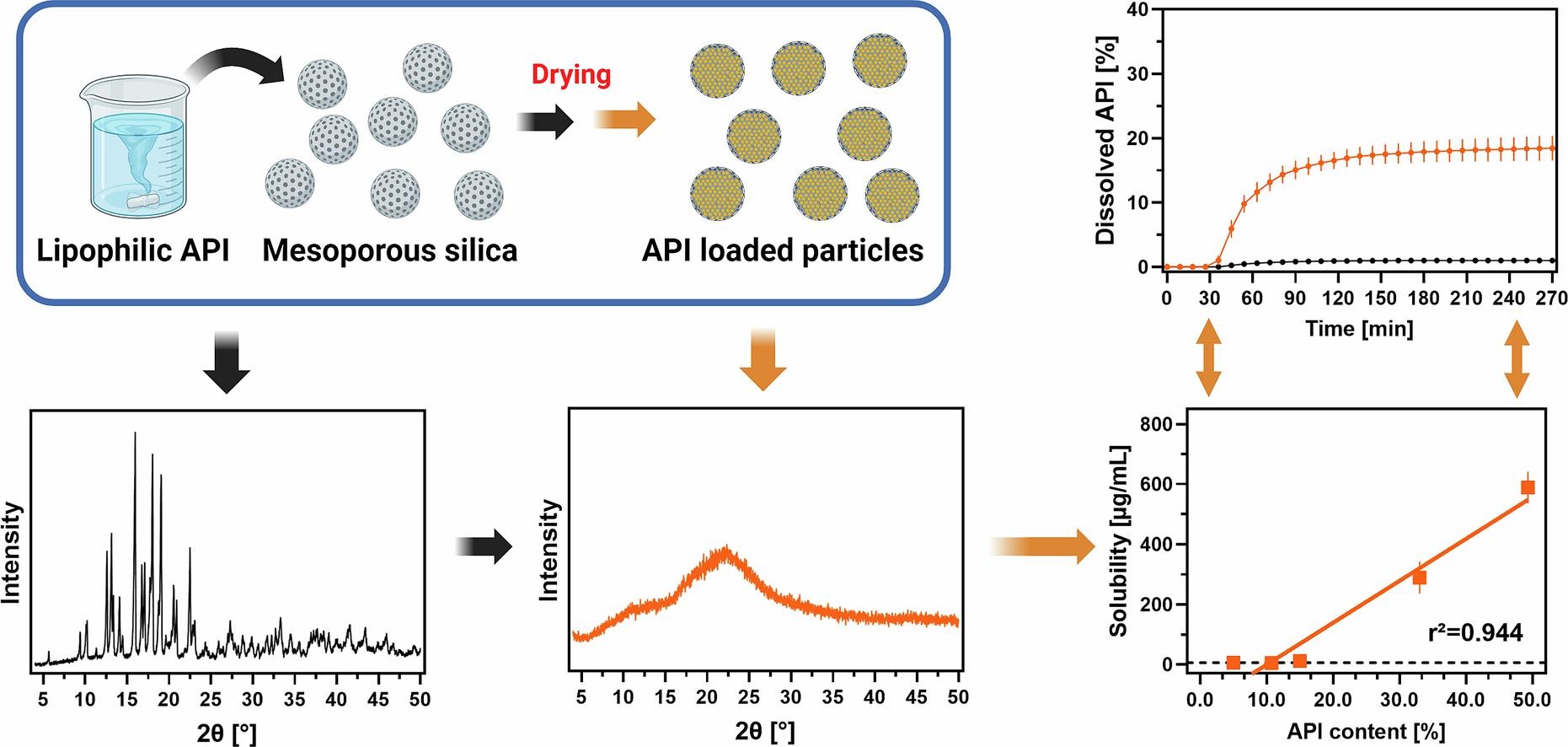
Loading poorly soluble active pharmaceutical ingredients (API) into mesoporous silica can enable API stabilization in non-crystalline form, which leads to improved dissolution. This is particularly beneficial for highly lipophilic APIs (log D7.4 > 8) as these drugs often exhibit limited solubility in dispersion forming carrier polymers, resulting in low drug load and reduced solid state stability. To overcome this challenge, we loaded the highly lipophilic natural products coenzyme Q10 (CoQ10) and astaxanthin (ASX), as well as the synthetic APIs probucol (PB) and lumefantrine (LU) into the mesoporous silica carriers Syloid® XDP 3050 and Silsol® 6035. All formulations were physically stable in their non-crystalline form and drug loads of up to 50 % were achieved.
Highlights
- Loading mesoporous silica is an excellent formulation approach for lipophilic APIs.
- Drug loads of up to 50% (w/w) could be achieved.
- Even at high drug loads all tested APIs remained in a non-crystalline form.
- Solubility and dissolution increased decisively with increasing drug load.
- In silico calculations explained performance differences between APIs.
At increasing drug loads, a marked increase in equilibrium solubility of the active ingredients in biorelevant medium was detected, leading to improved performance during biorelevant biphasic dissolution studies (BiPHa + ). Particularly the natural products CoQ10 and ASX showed substantial benefits from being loaded into mesoporous carrier particles and clearly outperformed currently available commercial formulations. Performance differences between the model compounds could be explained by in silico calculations of the mixing enthalpy for drug and silica in combination with an experimental chromatographic method to estimate molecular interactions.
Introduction
To achieve sufficient bioavailability, it is necessary to first dissolve an adequate dose fraction of the active pharmaceutical ingredient (API) in an aqueous gastrointestinal fluid (Barthe et al., 1999, Martinez and Amidon, 2002). High API concentrations in solution increase the driving force for absorption through the intestinal membrane (Taylor and Zhang, 2016). Subsequent to the absorption, the API enters systemic circulation and is transported to the relevant side of action where the biological activity can take place (Martinez and Amidon, 2002). Unfortunately, a decrease in water solubility limits the dissolution, absorption and hence bioavailability of active ingredients, whereby a high degree of compound lipophilicity and/or hydrophobicity are key factors (Ditzinger et al., 2019a). Compounds that have high Tm values and low to moderate log P values are commonly referred to as ‘brick-dust’ compounds, whereas those with low Tm values and high log P values are known as ‘grease balls’. The brick-dust compounds have limited solubility by their solid-state, where the breakdown of the crystal lattice is the most challenging step.
On the other hand, the grease balls have the solvation step in water as the primary obstacle for drug dissolution (Ditzinger et al., 2019a). This becomes particularly problematic considering that roughly 40–90 % of the APIs currently in development encounter solubility issues (Loftsson and Brewster, 2010, Ting et al., 2018). However, this problem is not only limited to synthetic APIs but also affects substances of natural origin. Especially tri- and tetraterpenes (e.g. astaxanthin and coenzyme Q10) experience reduced water solubility, attributed to their lipophilic isoprenoid chains (Atriya et al., 2023, Schulz et al., 2006). Absorption via the lymphatic pathway after incorporation into chylomicrons can be assumed to occur for this type of lipophilic natural products (Schulz et al., 2006).
Nevertheless, the lymphatic pathway also requires in situ micellization of the dissolved API with the aid of bile salts and absorption only occurs after emulsification (Managuli et al., 2018). Addressing this concern, several approaches have been suggested to improve absorption of highly lipophilic APIs, which includes lipid-based formulations targeting enhanced solubilization and an increase in absorption through the lymphatic pathway (Schulz et al., 2006). The surfactants and lipids contained in especially the self-emulsifying formulations promote integration of the API in micellar inclusion complexes even when only small solvent volumes are present (Neslihan Gursoy and Benita, 2004). However, the bioavailability improvement achieved by these formulations may sometimes be compromised, as the formation of stable micellar complexes can hinder intestinal absorption accompanied by the overall issue of limited dose strength of lipid-based systems.
One of the most preferred techniques, that is widely used for increasing solubility and bioavailability especially of poorly soluble synthetic APIs, is stabilizing the drug in its amorphous state. Amorphous materials have higher solubility and dissolve faster than their crystalline counterparts (Taylor and Zhang, 2016). Unlike the crystalline form, dissolving amorphous material does not require breaking of the crystal lattice structure so concentrations can be reached beyond the thermodynamic solubility (Singh and Van den Mooter, 2016).
Consequently, various strategies have been postulated to hinder recrystallization while maintaining the solubility benefits of an amorphous molecular structure (Brouwers et al., 2009). These approaches include co-grinding (Li et al., 2019a), co-amorphous formulations (Karagianni et al., 2018), preparation of polymer-based amorphous solid dispersions (ASD) (Baghel et al., 2016, Pöstges et al., 2022, Taylor and Zhang, 2016), or mesoporous silica-based systems (Hate et al., 2020, McCarthy et al., 2018, Price et al., 2019, Vraníková et al., 2020). Considering ASDs, polymeric excipients decrease molecular mobility of the API and thereby enable a stabilization in amorphous form, although this is thermodynamically unfavorable (Bookwala and Wildfong, 2023). Nonetheless, this strategy can pose a challenge for lipophilic active ingredients, as poor solubility in the polymer matrix results in limited drug loading and eventually formulation instability as high kinetic concentrations in the polymer matrix elevate the risk of recrystallization and phase separation during storage (Janssens and Van den Mooter, 2009, Wolbert et al., 2022).
Another proven technique for maintaining active ingredients in a non-crystalline form is using mesoporous silica particles as a carrier material (Ditzinger et al., 2019b, Niederquell et al., 2023). Drug loading can be achieved either with solvent-based techniques, for example incipient wetness impregnation or by solvent-free methods like drug melting or the use of supercritical fluids (Li et al., 2019b; Seljak et al., 2020). Mesoporous systems are known for their ability to inhibit the crystallization process of drugs by confining them in carrier pores, especially if the pore size is comparable to the critical nuclei size. Shen et al. (2017) suggested that a drug would remain in a non-crystalline form within the mesopores if the pore size were smaller than 12 times the molecular size of the API (Shen et al., 2017).
More refined estimates can be found in the literature based on the classical theory of homogeneous nucleation assuming that only nuclei larger than a critical nucleation size can grow and cause formulation instability (Descamps and Willart, 2018, Knapik et al., 2016, Vraníková et al., 2020). Furthermore, the molecular mobility of APIs incorporated in small enough pores is significantly lower than in the bulk (Vraníková et al., 2020). Consequently, there is not surrounding polymer matrix needed as in case of ASDs, which appears beneficial for enabling the use of highly lipophilic/ poorly soluble APIs. Comparable to ASDs, the non-crystalline form allows for a significantly higher release of active ingredients than the crystalline molecular form. In addition, Le et al. (2019) observed an increase in dissolution performance as drug load increased, even surpassing the theoretical monolayer capacity of Syloid® XDP 3050 used as a carrier (Le et al., 2019). Dening and Taylor, on the other hand, observed a notable decrease in diffusive flux for ritonavir loaded SBA-15 when the theoretical monolayer surface coverage was exceeded (Dening and Taylor, 2018). Accordingly, the effects of different drug loads on solubility and release of different APIs from mesoporous silica carriers has not yet been fully elucidated und further work is necessary.
The aim of our study was to explore how various drug loads of highly lipophilic (log D7.4 > 8) synthetic APIs (probucol and lumefantrine) and natural products (coenzyme Q10 and astaxanthin) impact the solubility and dissolution behavior of the active ingredients in various media. The term natural product refers to extracts of natural origin, which are composed of several structurally related but not identical active ingredients.
The astaxanthin enriched oleoresin contains more than 20 different mono- and diesters of astaxanthin. Thereby the sum of all individual components is referred to as “the active ingredient”. By comparison, synthetic APIs typically consist of only one molecular species with precisely known structural identity. Syloid® XDP 3050 (average pore diameter: 22.9 nm (Waters et al., 2018)) and Silsol® 6035 (average pore diameter: 6.0 nm (Cokenakes, 2021)) were used as mesoporous carriers. In addition, the suitability of this formulation principle for improving the biorelevant dissolution performance of the highly lipophilic and poorly soluble model APIs was investigated using a biphasic dissolution assay.
Materials
Coenzyme Q10 (CoQ10) (purity ≥ 99.1 %, CAS 303–98-0) from Abcr GmbH (Karlsruhe, Germany), astaxanthin (ASX) enriched Haematococcus pluvialis oleoresin (10.1 % ASX content) from BDI-BioLife Science GmbH (Hartberg, Austria), probucol (PB) (purity ≥ 99.7 %, CAS 23288–49-5) and lumefantrine (LU) (purity ≥ 99.6 %, CAS 82186–77-4), both from Swapnroop Drugs & Pharmaceuticals (Aurangabad, India), were used as model compounds. CoQ10 oily dispersion (Nature Made® CoQ10, Pharmavite®, Northridge, CA,
Read more
Marvin Benedikt Brenner, Matthias Wüst, Martin Kuentz, Karl G. Wagner, High loading of lipophilic compounds in mesoporous silica for improved solubility and dissolution performance, International Journal of Pharmaceutics, Volume 654, 2024, 123946, ISSN 0378-5173,
https://doi.org/10.1016/j.ijpharm.2024.123946.

