Mesoporous Silica as an Alternative Vehicle to Overcome Solubility Limitations
Abstract
Introduction
During the preclinical drug investigations, an early toxicology assessment is required to ensure safe entry into human clinical studies [1]. This typically includes investigations in different preclinical species; however, these differ in physiologies and, thus, in requirements regarding the route of administration and the type of formulation [2]. Particularly for the development of poorly soluble drugs, providing an appropriate vehicle covering all requirements is an immense challenge for pharmaceutical scientists. In general, the oral administration is the most common route, which means that this must be considered during toxicology studies. Enabling sufficient exposure is crucial to identify adverse effects in conjunction with establishing a safety window for human administration.
Solutions are the preferred formulations due to the easy handling (e.g., dose escalation) and the elimination of dissolution dependency [2]. However, in contrast to pharmacokinetic and pharmacodynamic studies, the dose levels required for a toxicology study exceed the therapeutically relevant dose many times over, with the aim of observing adverse effects. For drugs with low toxicity, the administered dose potentially increases to a maximum of 1000 mg/kg of bodyweight, which further limits the assortment of appropriate study vehicles [3]. Ideally, the vehicle should use a solubility-enhancing formulation strategy to provide improved exposure and, thus, high bioavailability. In addition, the successful evaluation of toxic effects is dependent on the tolerability of the respective vehicle, which is, in turn, dependent on species-specific factors such as route of administration tolerances, concentrations, volumes, dosing regimens, and study duration [4].
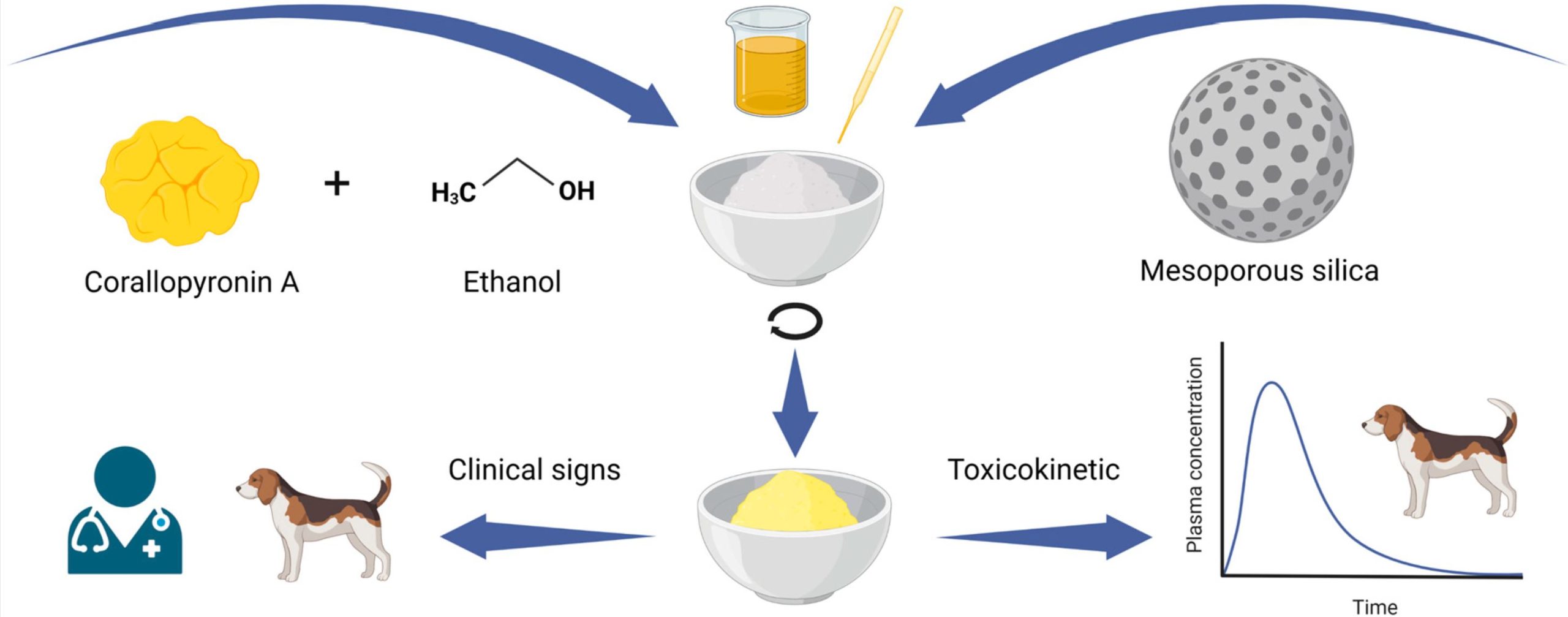
For example, the commonly used solvent polyethylene glycol (PEG) 400 is known to exhibit gastrointestinal adverse effects like watery feces or emesis following oral administration in dogs [5]. Enabling formulations like amorphous solid dispersions (ASD) are commonly used to improve bioavailability. However, high polymer quantities for several days can result in agglomeration and the formation of pharmacobezoars, leading to a fatal obstructive ileus [6]. The objective of this work was to identify an alternative vehicle for the toxicology studies in Beagle dogs for the low-toxicity anti-infective corallopyronin A (CorA), which is currently under late preclinical development for parasitic filarial nematode and bacterial infections [7]. According to the Guideline on Repeated Dose Toxicity, two different species of mammals should be used in preclinical drug development [3]. Previous toxicological studies were conducted in Wistar rats, representing the rodent species. For the toxicological investigation in a non-rodent species, Beagle dogs were selected.
The combination of the poor aqueous solubility of CorA (91.13 µg/mL at pH 6.5) and the required high dose levels (150–750 mg/kg) excluded many common vehicles for toxicology studies [7]. Moreover, the waxy consistency of the fully amorphous CorA (glass transition temperature of 5 °C) did not allow for the suspending of the drug substance as a homogeneous suspension [7]. In recent years, mesoporous silica has been proven to be an easy-to-use powdered intermediate that increases the bioavailability of poorly soluble drugs. In contrast to ASDs, the risk of pharmacobezoar formation was assumed to be lower due to the mineral and inert character of mesoporous silica. Therefore, we aimed to introduce the mesoporous silica formulation principle as a drug carrier for toxicological studies of CorA and a potential option for future challenging drug candidates.
Download the full article as PDF here: Mesoporous Silica as an Alternative Vehicle to Overcome Solubility Limitations
or read it here
Materials
CorA (purity ≥ 90%) was produced by the Helmholtz Centre for Infection Research (Braunschweig, Germany) [7]. Mesoporous silica, Syloid® XDP 3050, was kindly provided by Grace GmbH (Worms, Germany). Absolute ethanol (purity: 99.8%) was purchased from Carl Roth GmbH & Co. KG (Karlsruhe, Germany). NATROSOL® 250 G Pharm was obtained from Caesar & Loretz GmbH (Bonn, Germany). For toxicokinetic studies plastic feeding tubes, sterile plastic syringes and needles for blood samplings were from B. Braun (Melsungen, Germany), blood sampling tubes Vacuette® K2-EDTA were from Greiner Bio-One GmbH (Frickenhausen, Germany), and plastic reaction tubes were from Eppendorf SE (Hamburg, Germany). For the analytics of the plasma samples via HPLC, LC-MS grade acetonitrile, and water were purchased from Bernd Kraft GmbH (Duisburg, Germany), and LC-MS grade ammonium acetate from Merck KGaA (Darmstadt, Germany).
Becker, T.; Heitkötter, J.; Krome, A.K.; Schiefer, A.; Pfarr, K.; Ehrens, A.; Grosse, M.; Sandargo, B.; Stammberger, I.; Stadler, M.; et al. Mesoporous Silica as an Alternative Vehicle to Overcome Solubility Limitations. Pharmaceutics 2024, 16, 386. https://doi.org/10.3390/pharmaceutics16030386
Read more interessting articles on Mesoporous Silica here:
- High loading of lipophilic compounds in mesoporous silica for improved solubility and dissolution performance
- Aptamer-modified chitosan-capped Mesoporous silica nanoparticles for co-delivery of cytarabine and daunorubicin in Leukemia
- A dual catalytic functionalized hollow mesoporous silica-based nanocarrier coated with bacteria-derived exopolysaccharides for targeted delivery of irinotecan to colorectal cancer cells


