Influence of In Situ Calcium Pectinate Coating on Metoprolol Tartrate Pellets for Controlled Release and Colon-Specific Drug Delivery
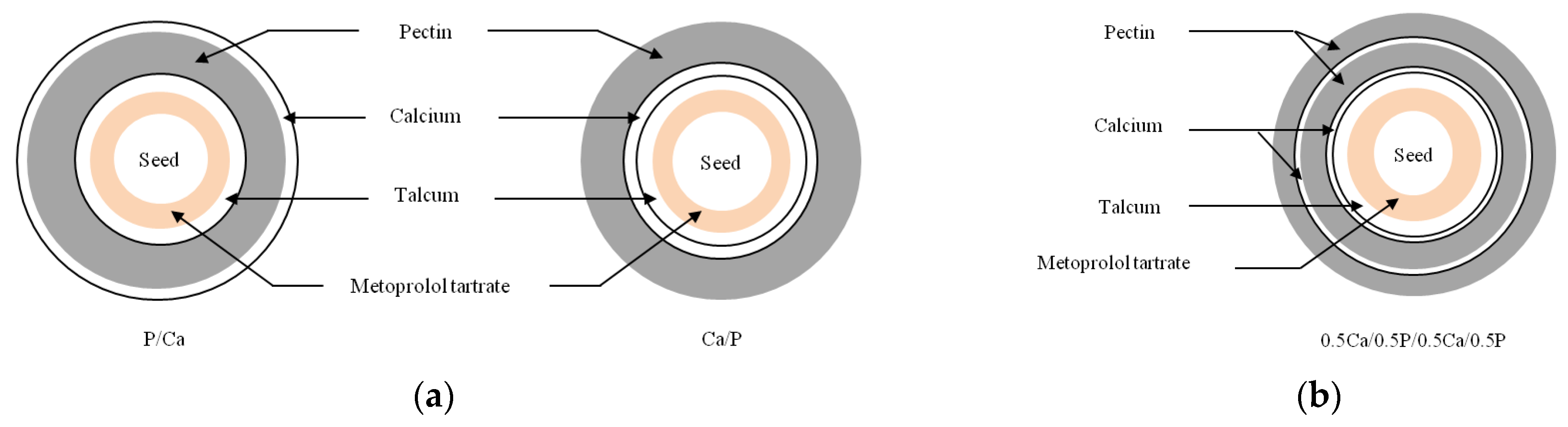
In situ calcium pectinate-coated pellets were proposed by applying an alternate coating method to drug-layered pellets to achieve colon-specific drug delivery. Solution layering of metroprolol tartrate, a water-soluble model drug, on inert core pellets was achieved using a centrifugal granulator followed by successive alternate coating with pectin and calcium chloride layers using a fluidized bed bottom spray coater. The effect of the coating sequence on the drug release was studied in phosphate buffer pH 7.4 and 6.0. These test conditions were used to mimic the physiological environments in the distal small intestine and proximal colon, respectively. The results showed that the in situ calcium pectinate layer was successfully generated from the alternate coating of pectin and calcium layers after hydration to form gelation, which was able to control the drug release. The coating sequence played an important role in the drug release. The outermost pectin layer tended to retard the drug release whilst the outermost calcium layer accelerated the release regardless of the number of coating layers. These findings indicate that the release behavior followed the Higuchi model, with the drug release from the coated pellets described by a diffusion control mechanism. It is concluded that the success of the in situ calcium pectinate-coated pellets in controlling the drug release is due to the coating of the outermost layer with pectin and the maintenance of the optimum ratio of calcium to pectin upon hydration.
Continue reading here
About this article: Wanasawas, Pimphaka, Ampol Mitrevej, and Nuttanan Sinchaipanid. 2022. “Influence of In Situ Calcium Pectinate Coating on Metoprolol Tartrate Pellets for Controlled Release and Colon-Specific Drug Delivery” Pharmaceutics 14, no. 5: 1061. https://doi.org/10.3390/pharmaceutics14051061
Materials
Metoprolol tartrate (Karinco, Milano, Italy) was used as a model drug. Microcrystalline cellulose (MCC) seeds (Cellets® 700), kindly provided by Pharmatrans Sanaq AG, Allschwil, Switzerland, were used as core materials. Hydroxypropyl methylcellulose (HPMC) (Methocel® E15LV, Colorcon, Dartford, UK) was applied as a binder and sub-coating film layer. Pectin from citrus fruits (8.1% methoxy content) and calcium chloride dihydrate were supplied by Sigma (St. Louis, MO, USA) and Carlo Erba Reagenti (Cornaredo, Italy), respectively. Silicone emulsion (Dow Corning® Antifoam 1614 DCS, AJAX Finechem, Sydney, Australia) was used as an antifoaming agent. Other excipients, including talcum, polyethylene glycol (PEG) 6000, and ethanol, were all of pharmaceutical or analytical grades.
Read more on Binder – Pharmaceutical Excipients here:


