MicroCoat™: A Breakthrough in Oral Multiparticulate Formulation Development
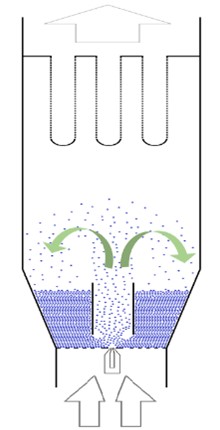
Oral multiparticulate systems are often the ‘go to’ solution for flexible dosing, especially in cases where a degree of functionality, such as taste masking or modified release, is required from the formulation. One of the preferred methods of adding functionality to multiparticulates is by fluid bed coating (FBC) whereby multiparticulates are fluidised with heated air and simultaneously spayed with materials designed to provide a specific function (Figure 1). This is due to efficient product mixing and drying in addition to the uniform coatings which can be achieved.
Conventional multiparticulates sized 500 µm to 1 mm are commonly processed by FBC, however, due to the size these can feel gritty in the mouth which can lead to non-compliance and can trigger chewing which can cause damage to the coating, compromising its functionality. In a world where patient centricity is at the core of medicine development, the acceptability of functional multiparticulates must be considered during design of the final drug product and in this regard, there is room for improvement by reducing the multiparticulate size. Producing a final dosage form with a multiparticulate diameter smaller than 250 µm (using starting material <200 µm in diameter) can eliminate the gritty mouth feel and improve acceptability[1].
Unfortunately, once you breach below the 200 µm threshold for the starting multiparticulate diameter, considered as micropellets, processability becomes increasingly challenging. This is due to their low mass and high surface area to volume ratio which elevate micropellet cohesivity relative to conventional multiparticulates and causes aggregation of micropellets in various regions within the coater, and to each other (Figure 2).
Figure 2. Outcomes from a conventional FBC process whereby Cellets® 100 (median diameter ≈150 µm) were coated with a benchtop Würster coater (Mini-Glatt®) using an aqueous polymer dispersion. Shows significant micropellet aggregation in the ‘down flow bed’ (left), drying zone (centre) and micropellet agglomeration (right).
These processing challenges can be overcome by reducing the spray rates during coating; however, this prolongs the overall manufacturing process and increases overall cost of manufacturing. Fluid Pharma have developed a novel FBC innovation, MicroCoat™, which facilitates the flow of micropellets during the spraying process without reducing spray rate and provides in situ stabilisation of the micropellets to reduce agglomeration and significantly increase yield. In a direct comparison to the trial pictured above, the implementation of the MicroCoat™ technology increased yield from 55 to 99 % (Figure 3).

Figure 3. Outcomes from a FBC process following the implementation of the MicroCoat™ technology whereby Cellets® 100 (median diameter ≈150 µm) were coated with a benchtop Würster coater (Mini-Glatt®) using an aqueous polymer dispersion. Shows no micropellet aggregation in the coating chamber and non-agglomerated micropellets after coating was completed.
Fluid Pharma have leveraged the MicroCoat™ technology to develop patient centric oral solid and liquid dosage forms comprising coated micropellets. These range from micropellets in stick packs, orally disintegrating tablets (ODT’s) and dry powders for reconstitution. The specific composition of the coatings can be changed to provide taste masking, enteric protection and sustained release profiles, depending on the need from the final product and showcases the breadth of possibilities for MicroCoat™ as a formulation platform for oral patient centric formulations.
Download the article as PDF here MicroCoat™: A Breakthrough in Oral Multiparticulate Formulation Development
or read it here
Source: FluidPharma, Kavil Patel, article “MicroCoat™: A Breakthrough in Oral Multiparticulate Formulation Development”
Interested in more information about MicroCoat™ or Fluidpharma?


